Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
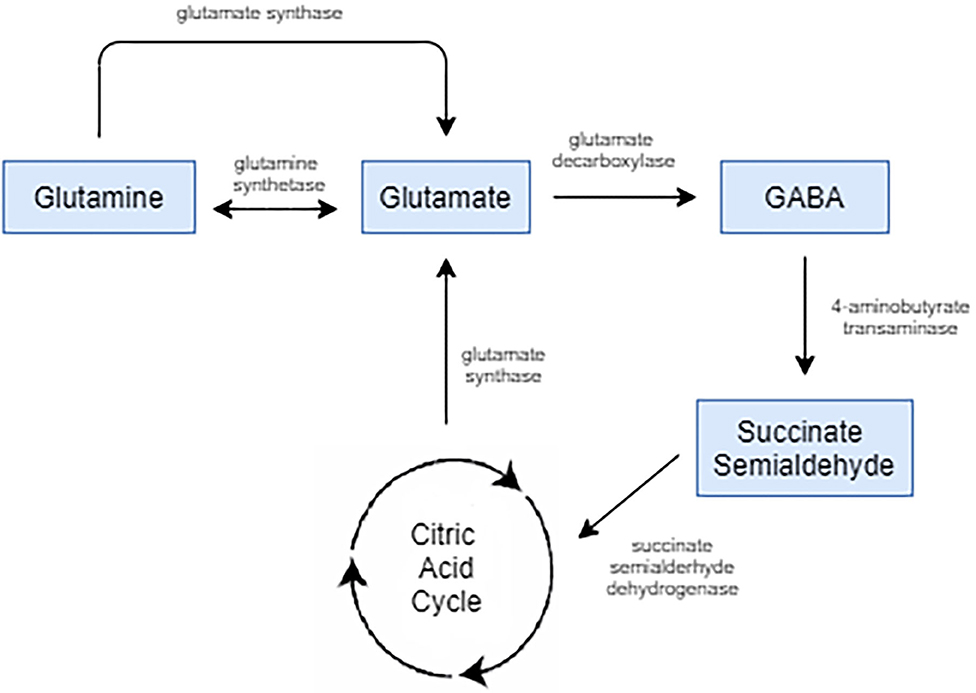 |  |
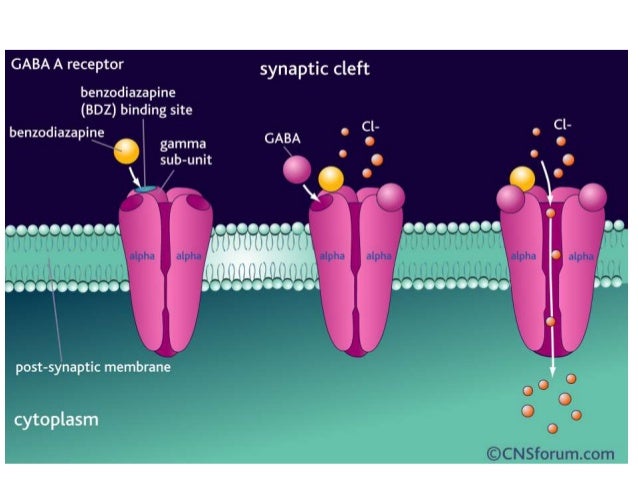
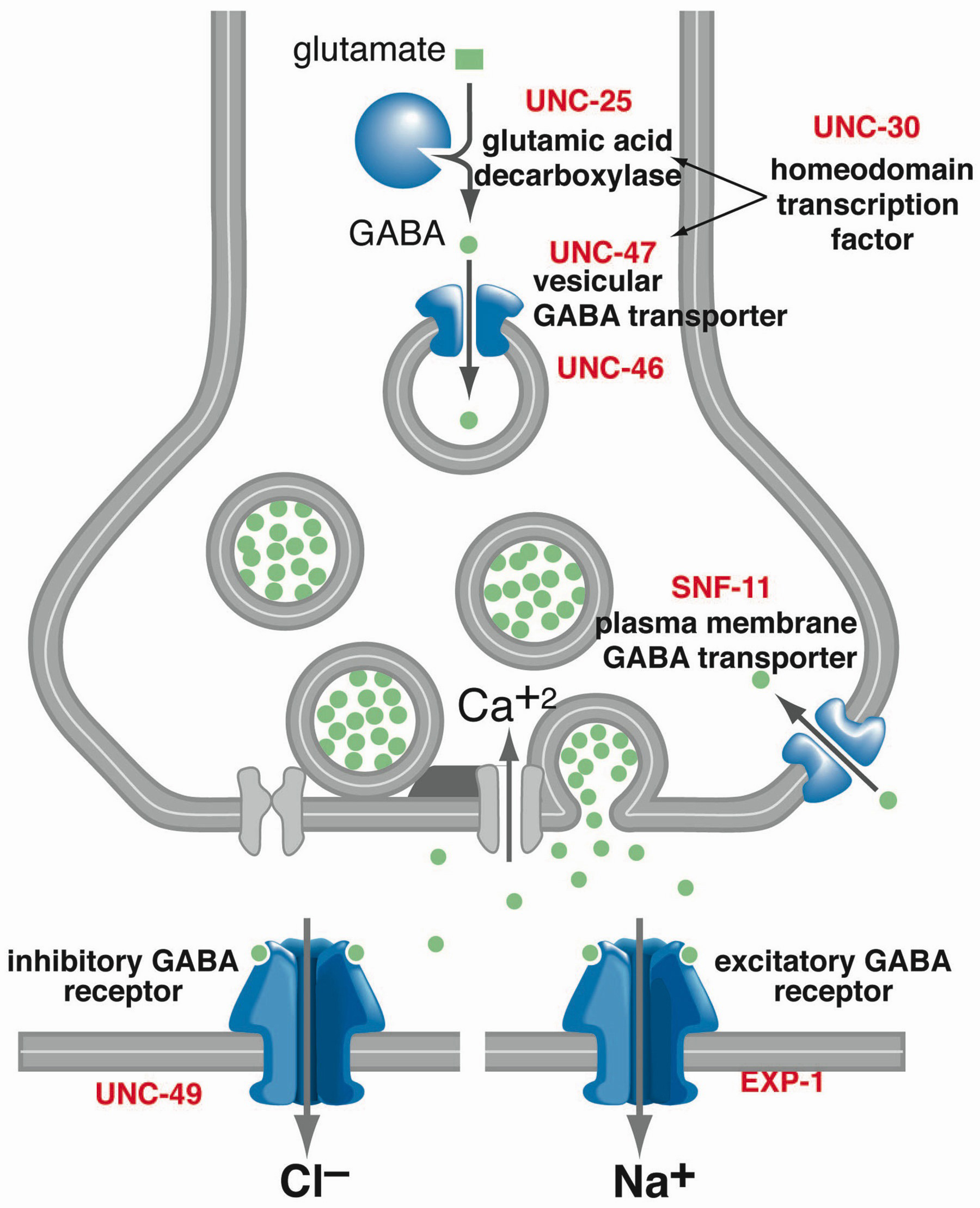
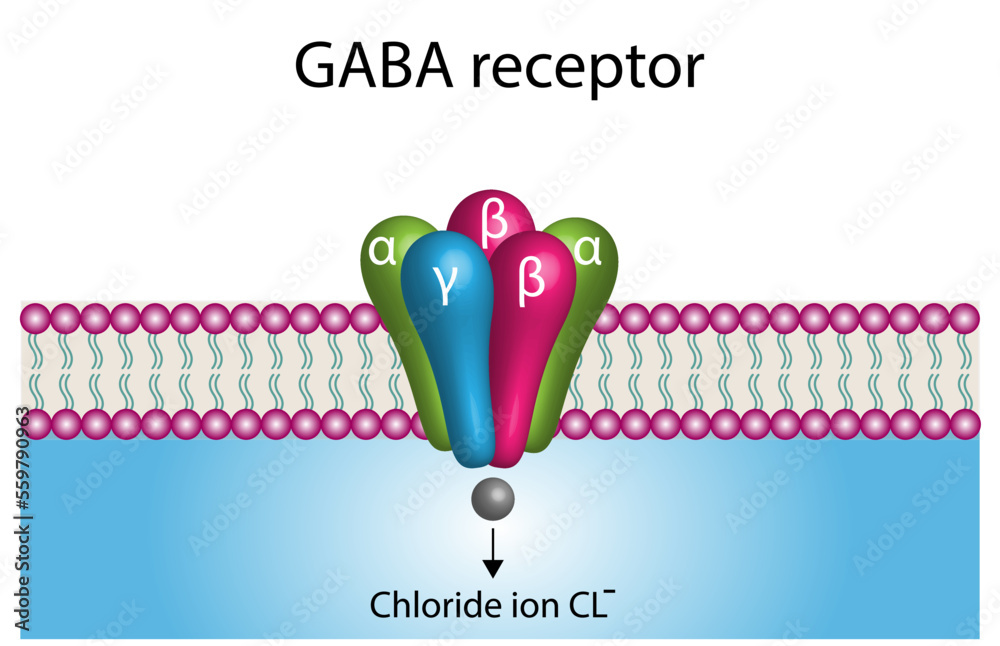
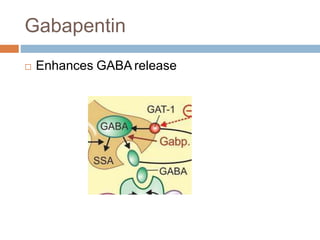
Gabapentin Extended-Release Tablets. Advertisement. An unusual or allergic reaction to gabapentin, other medications, foods, dyes, or preservatives; Note: Gabapentin is suggested by some experts as an alternative when first-line agents cannot be used (Johnson 2019; VA/DoD 2015). Gabapentin may be misused by some patients with substance use disorders; evaluate for risk and signs of addiction and dependence (Mersfelder 2016). Alcohol withdrawal, mild (alternative agent) (off-label use): Horizant (gabapentin enacarbil) is an extended release tablet used to treat restless legs syndrome and for the pain from having shingles (postherpetic nerve pain). Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older. Warnings γ-Amino butyric acid (GABA), the prototypical inhibitory neurotransmitter of the central nervous system, plays an important role in sensory processing, and analgesic effects of gabapentin may reflect modulation of GABA release. Gabapentin’s actions on GABA release are controversial and likely depend on the site in the central nervous system. Gabapentin, marketed for the treatment of seizures and neuropathic pain, has been shown to increase in vivo GABA concentration in the brain of both rodents and humans. Gabapentin effects on Despite its design and intended effect, gabapentin does not interact with GABA receptors, GABA uptake, or metabolism. It may influence synthesis or release of GABA, and it increases GABA concentrations in certain regions of the brain. Gabapentin increases GABA release from rat striatal brain slices in vitro (Götz et al., 1993), and this action is prevented by the GABA A antagonist, bicuculline. Child 6–11 years 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose maximum to be given in 3 divided On the other hand, Gabapentin is a medication that is structurally similar to GABA but does not directly bind to GABA receptors. Instead, it modulates the release of certain neurotransmitters, such as glutamate, to reduce nerve excitability and alleviate pain or seizures. Immediate-release: Initial dose: Day 1: 300 mg orally once Day 2: 300 mg orally 2 times day Day 3: 300 mg orally 3 times a day. Titrate dose as needed for pain relief; Maintenance dose: 900 to 1800 mg/day orally in 3 divided doses Maximum dose: 1800 mg per day Extended-release: Gralise (gabapentin) 24-hour extended-release tablets: Initial dose: An extended-release form of gabapentin is also FDA-approved to treat PHN. Another extended-release form of gabapentin is FDA-approved to treat restless legs syndrome. This condition causes unpleasant or uncomfortable sensations in the legs and an irresistible urge to move them around, especially at night, which disrupts sleep. Gabapentin is a GABA agonist and anticonvulsant that increases GABA concentrations in the central nervous system, possibly via inhibition of GABA-transaminase (Cai et al., 2012). While more commonly trialed in combination with flumazenil, as described above, one double-blind RCT ( Heinzerling et al., 2006 ) examined gabapentin as an individual Gabapentin increases non-synaptic GABA responses from neuronal tissues in vitro. In vitro, gabapentin reduces the release of several mono-amine neurotransmitters. Gabapentin prevents pain responses in several animal models of hyperalgesia and prevents neuronal death in vitro and in vivo with models of the neurodegenerative disease amyotrophic Gabapentin is a gabapentinoid, which acts as an inhibitor of the α2δ subunit-containing voltage-dependent calcium channels (VDCCs) that are linked to neurotransmitter release. Gabapentin binds with high affinity to the α 2 δ VDCCs [6], which is considered the primary target of the drug. Here, we postulated that gabapentin increases expression of δ subunit-containing GABA A (δGABA A) receptors that generate a tonic inhibitory conductance in multiple brain regions including the cerebellum and hippocampus. Gabapentin is a structural analog of the inhibitory neurotransmitter γ-aminobutyric acid (GABA). Its anticonvulsant, analgesic and anxiolytic properties suggest that it increases GABAergic inhibition; however, the molecular basis for these effects is unknown as gabapentin does not directly modify GABA type A (GABA A) receptor function, nor does it modify synaptic inhibition. Gabapentin enhanced expression of δGABA A receptors and increased a tonic inhibitory conductance in neurons. This increased expression likely contributes to GABAergic effects as gabapentin caused ataxia and anxiolysis in wild-type mice but not δ subunit null-mutant mice. Gabapentin, marketed for the treatment of seizures and neuropathic pain, has been shown to increase in vivo GABA concentration in the brain of both rodents and humans. Gabapentin effects on glutamate are not known. Gabapentin, marketed for the treatment of seizures and neuropathic pain, has been shown to increase in vivo GABA concentration in the brain of both rodents and humans. Gabapentin effects on glutamate are not known. By the early 1970s, it was appreciated that there are two main classes of GABA receptors, GABA A and GABA B and also that baclofen was an agonist of GABA B receptors. Gabapentin was designed, synthesized and tested in mice by researchers at the pharmaceutical company Goedecke AG in Freiburg, Germany (a subsidiary of Parke-Davis ).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |