Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |
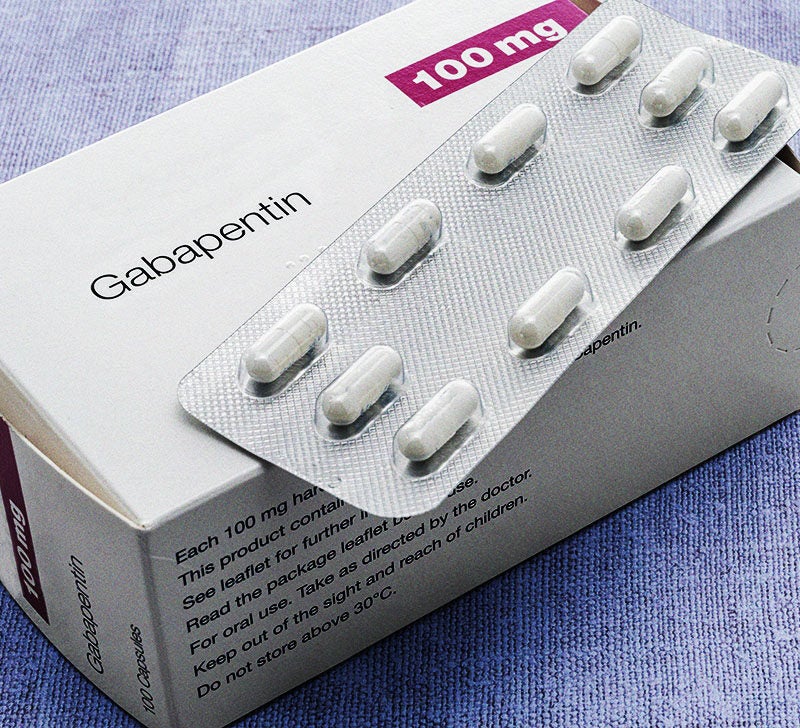
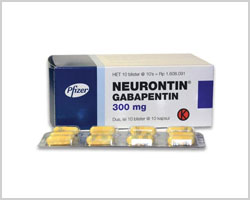
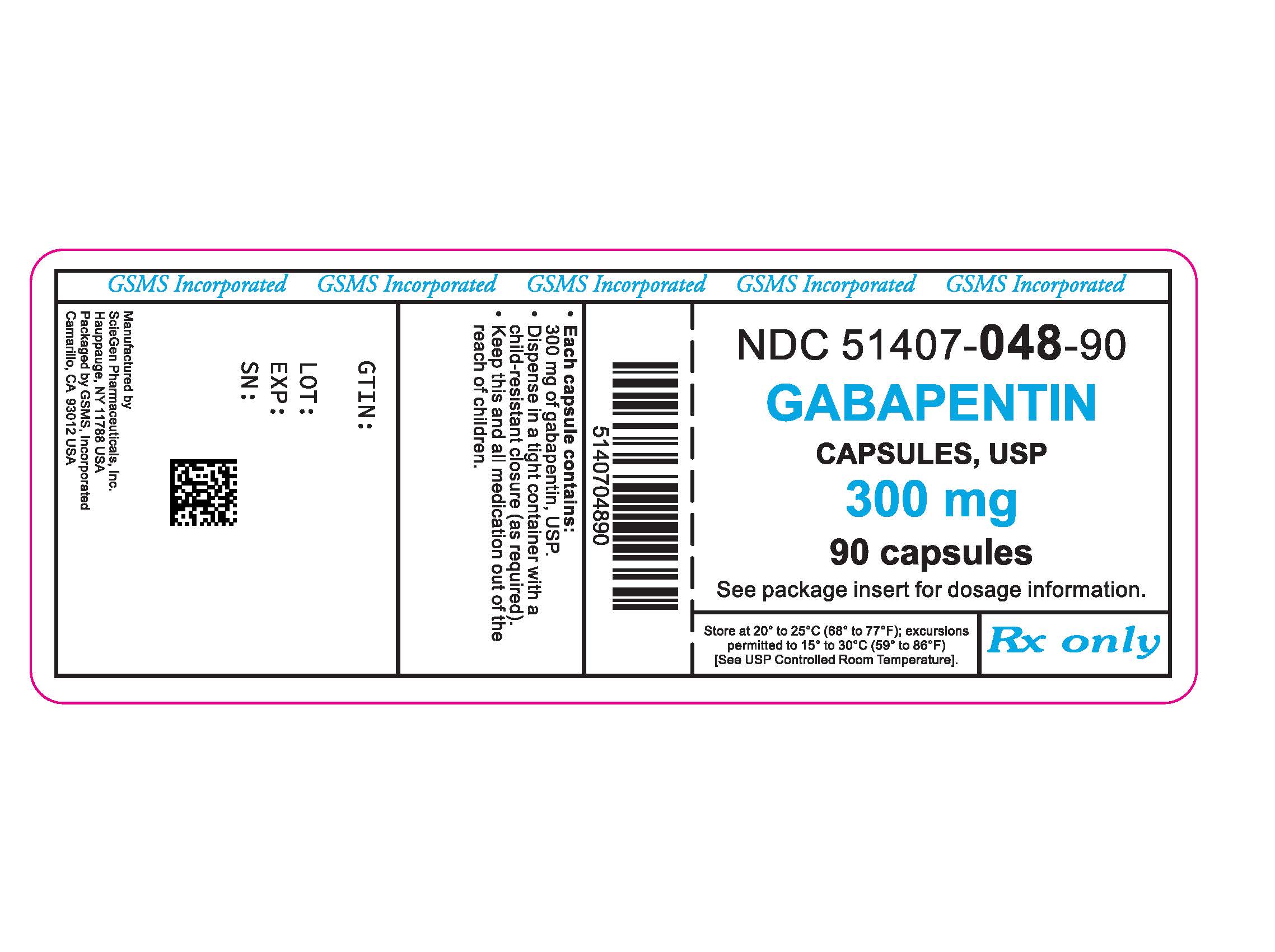
Is there a generic version of Neurontin available? Yes. The following products are equivalent to Neurontin: gabapentin capsule;oral. Manufacturer: ACTAVIS ELIZABETH Approval date: September 12, 2003 Strength(s): 100MG , 300MG , 400MG ; Manufacturer: ALKEM Approval date: December 17, 2010 Strength(s): 100MG , 300MG , 400MG Generic name: gabapentin Dosage form: tablet Drug class: Marketing Start Date Marketing End Date; 1: NDC:74157-013-90: 90 in 1 BOTTLE; Type 0: Not a Combination Generic Gralise Availability. Last updated on Mar 13, 2025. Gralise is a brand name of gabapentin, approved by the FDA in the following formulation(s): GRALISE (gabapentin - tablet;oral) Manufacturer: ALMATICA Approval date: January 28, 2011 Strength(s): 300MG , 600MG ; Manufacturer: ALMATICA Approval date: April 18, 2023 January 25, 2024 - Zydus launched AB-rated generic versions of Almatica’s Gralise (gabapentin) 300 mg and 600 mg tablets. Store at room temperature between 15 and 30 degrees C (59 and 86 degrees F). Get rid of any unused medication after the expiration date. This medication may cause accidental overdose and death if taken by other adults, children, or pets. Mix any unused medication with a substance like cat litter or coffee grounds. Gabapentin was first approved for use in the United Kingdom in 1993. [16] . It has been available as a generic medication in the United States since 2004. [17] . It is the first of several other drugs that are similar in structure and mechanism, called gabapentinoids. What is Gabapentin generic date? Gabapentin generic date is a highly effective medication that has been proven to alleviate various types of pain, including neuropathic pain, postherpetic neuralgia, and fibromyalgia. Its unique formula targets the root cause of pain, providing long-lasting relief. The benefits of Gabapentin generic date. 1. Neurontin (gabapentin) is prescribed for the treatment of seizures and alternative forms of pain. Neurontin (gabapentin) is additionally prescribed along side alternative medicine for the treatment and management of postherpetic neuralgia, a sort of seizure and nerve pain led to by herpes virus or shingles. Horizant is a drug marketed by Azurity and is included in one NDA. There are five patents protecting this drug and one Paragraph IV challenge. This drug has one hundred and forty-six patent family members in twenty-six countries. The generic ingredient in HORIZANT is gabapentin enacarbil. There are twenty-nine drug master file entries for this Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older. Gabapentin can cause life-threatening breathing problems, especially if you already have a breathing disorder or if you use other medicines that can make you drowsy or slow your breathing. Gabapentin, a commonly prescribed medication for treating nerve pain Gabapentin and pregabalin are both medications that belong to the class of antiepileptic drugs. While they have similar uses and mechanisms of action, there are some key differences between Are you suffering from the negative side effects of taking Adderall? On January 25, 2024, Zydus launched AB-rated generic versions of Almatica’s Gralise (gabapentin) 300 mg and 600 mg tablets. — Gralise is also available as 450 mg, 750 mg and 900 mg tablets. Gralise is approved for the management of postherpetic neuralgia (PHN). Gabapentin is a medication that treats nerve pain by calming overactive nerves in your body. It may also prevent and control seizures in people with epilepsy. You can take this medication by mouth with a glass of water. Talk to your provider about medications you currently take to avoid drug interaction. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Gabapentin generic names are affordable options to the brand name Gabapentin. These generic versions offer the same therapeutic benefits at a lower cost, making them a cost-effective alternative for patients. Generic Gabapentin is widely available in different formulations such as tablets, capsules, and oral solutions. This variety allows The first generic version for Gabapentin was by Actavis Elizabeth Llc and was approved on Sep 12, 2003. And the latest generic version is by Strides Pharma Global Pte Ltd and was approved on Dec 20, 2024. Given below is the list of companies who have filed for Gabapentin generic, along with the locations of their manufacturing plants worldwide. Generic Horizant Availability. Last updated on Mar 13, 2025. Horizant is a brand name of gabapentin enacarbil, approved by the FDA in the following formulation(s): HORIZANT (gabapentin enacarbil - tablet, extended release;oral) Manufacturer: AZURITY Approval date: April 6, 2011 Strength(s): 600MG ; Manufacturer: AZURITY Approval date: December Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Gabapentin is indicated for the management of postherpetic neuralgia. Gabapentin is not interchangeable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration. Gabapentin’s anticipated release date is in the first quarter of 2024.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |