Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 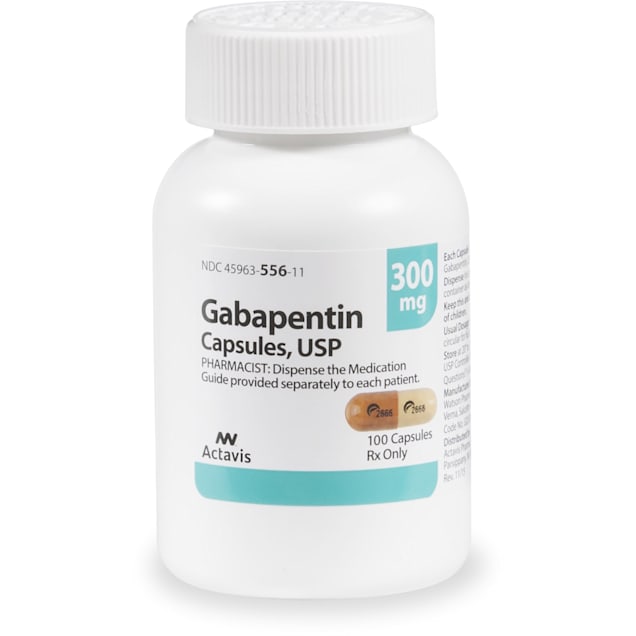 |
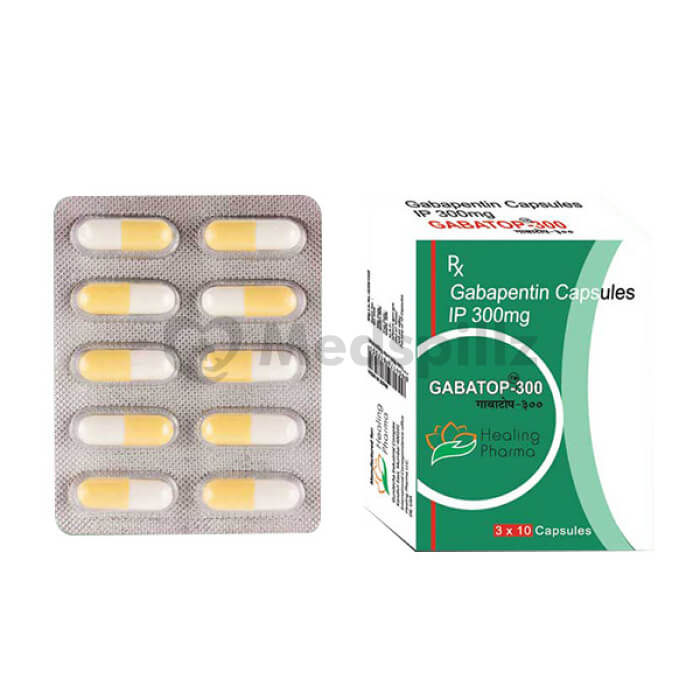
Gabapentin generic names are affordable options to the brand name Gabapentin. These generic versions offer the same therapeutic benefits at a lower cost, making them a cost-effective alternative for patients. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Generic Horizant Availability. Last updated on Mar 13, 2025. Horizant is a brand name of gabapentin enacarbil, approved by the FDA in the following formulation(s): HORIZANT (gabapentin enacarbil - tablet, extended release;oral) Manufacturer: AZURITY Approval date: April 6, 2011 Strength(s): 600MG ; Manufacturer: AZURITY Approval date: December Generic Release of Gralise (Gabapentin tablets) will be launched in the First Quarter of 2024. Released: 01/10/2024 . Generic Release of Gabapentin Extended-Release Tablets. temperature between 15 and 30 degrees C (59 and 86 degrees F). Get rid of any unused medication after the expiration date. January 25, 2024 - Zydus launched AB-rated generic versions of Almatica’s Gralise (gabapentin) 300 mg and 600 mg tablets. A generic version of gabapentin first became available in the United States in 2004. [17] An extended-release formulation of gabapentin for once-daily administration, under the brand name Gralise, was approved in the United States for the treatment of postherpetic neuralgia in January 2011. [117] [118] What is drug gabapentin for; Gabapentin capsules usp 100mg; Gabapentin ratiopharm 600; Mylan gabapentin 100mg; Gabapentin 600 mg side effcets; Online Pharmacy 2000 Zydus is the first company to receive final approval for generic Gabapentin Tablets (Once-Daily), 300 mg and 600 mg. Following approval, the product will be launched immediately in the US. Gabapentin is indicated for the management of Postherpetic Neuraligia (PHN). On January 25, 2024, Zydus launched AB-rated generic versions of Almatica’s Gralise (gabapentin) 300 mg and 600 mg tablets. — Gralise is also available as 450 mg, 750 mg and 900 mg tablets. Gralise is approved for the management of postherpetic neuralgia (PHN). Compare prescription drug prices and find coupons at more than 70,000 US pharmacies. Save up to 80% instantly! A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg GABARONE capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Generic name: gabapentin [ GA-ba-PEN-tin ] Brand names: Gralise , Horizant, Neurontin , Gabarone Dosage forms: oral capsule (100 mg; 300 mg; 400 mg), oral solution (250 mg/5 mL), oral tablet (600 mg; 800 mg), show all 4 dosage forms oral tablet, extended release (300 mg/24 hours; 450 mg/24 hours; 600 mg/24 hours; 750 mg/24 hours; 900 mg/24 Immediate-release forms of gabapentin (Neurontin, generic gabapentin) can be taken with or without food. The longer-acting forms of gabapentin (Horizant, Gralise) should be taken with food or a meal to help improve the absorption of the medicine. Camber already offers Gabapentin Tablets and Capsules (generic for Neurontin®). Generics for Gralise® and Neurontin® are not interchangeable. Gabapentin Tablets (generic for Gralise®) are indicated for the management of Postherpetic Neuralgia (PHN). Gabapentin Tablets (generic for Gralise®) 300 mg and 600 mg are available in 90 count bottles. Food and Drug Administration Approval Date: January 28, 2011 Product Launch Date: October 10, 2011 Overview/Summary Gralise® (gabapentin) is a new once-daily formulation of the antiepileptic drug gabapentin that is Food and Drug Administration (FDA)-approved for the management of postherpetic neuralgia (PHN).1 Generic Gralise Availability. Last updated on Mar 13, 2025. Gralise is a brand name of gabapentin, approved by the FDA in the following formulation(s): GRALISE (gabapentin - tablet;oral) Manufacturer: ALMATICA Approval date: January 28, 2011 Strength(s): 300MG , 600MG ; Manufacturer: ALMATICA Approval date: April 18, 2023 Generic name: gabapentin Dosage form: Extended Release Tablets Previous Name: DM-1796 Company: Depomed, Inc. Treatment for: Postherpetic Neuralgia. Gralise (gabapentin) is an extended release, tablet formulation of the approved antiepileptic agent gabapentin, indicated for the once-daily treatment of post-herpetic neuralgia (PHN). Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Gabapentin’s anticipated release date is in the first quarter of 2024.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |