Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
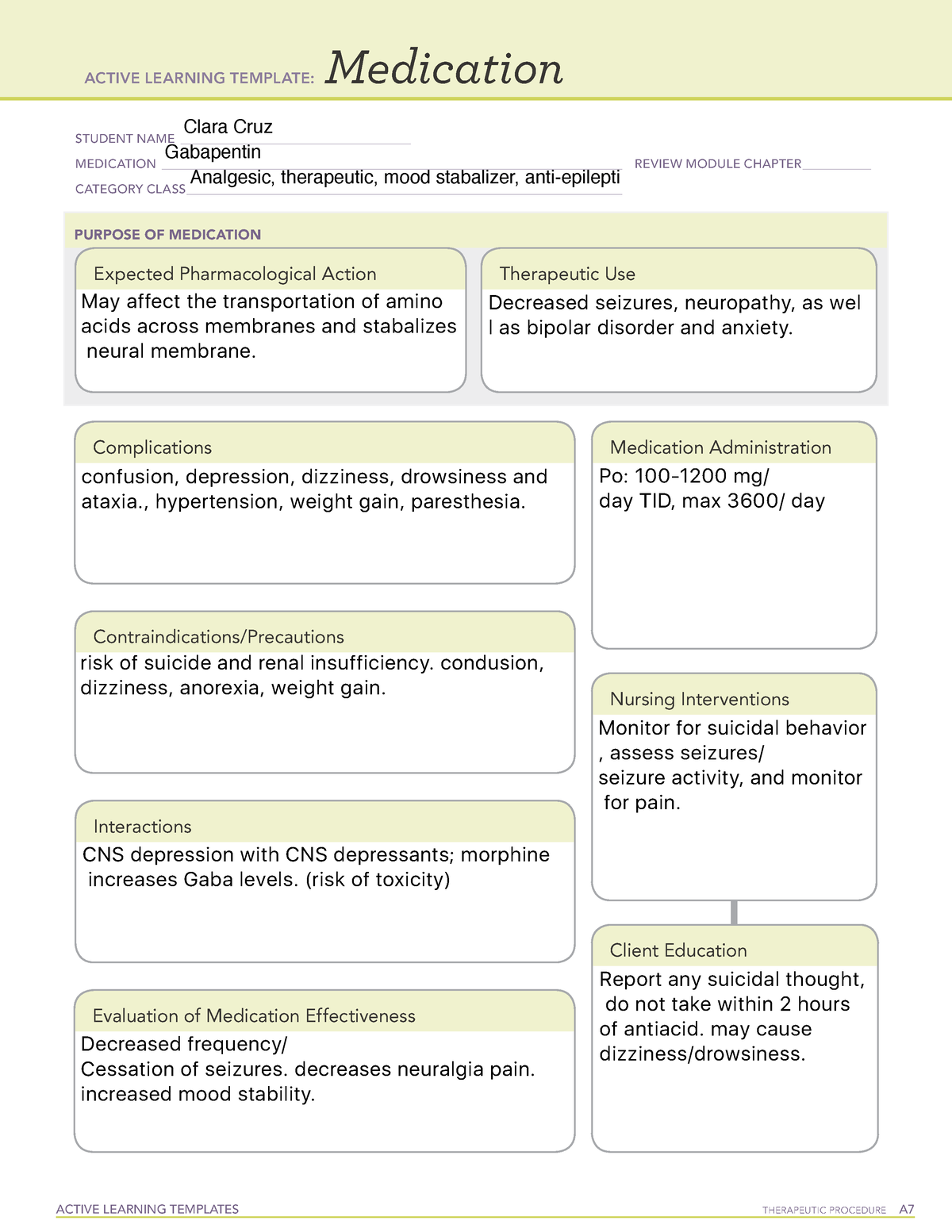
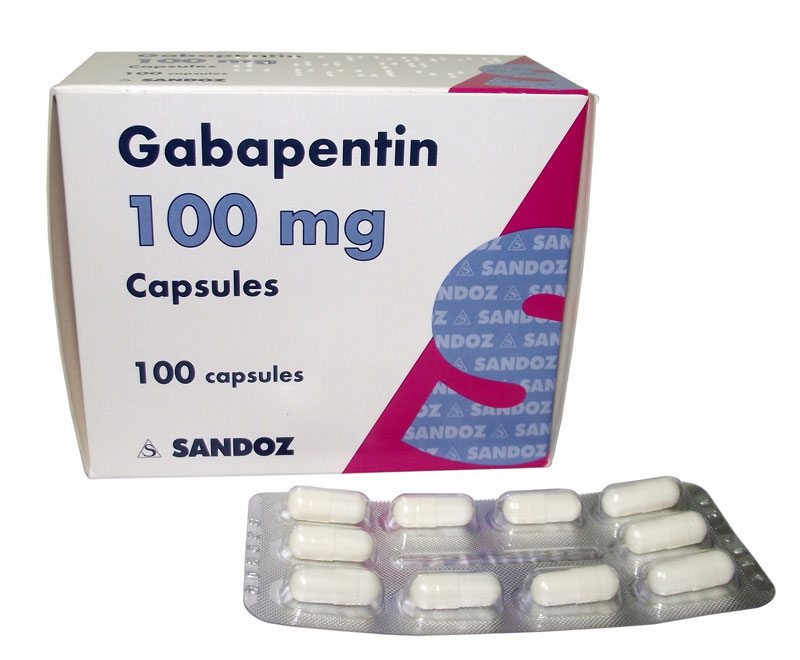
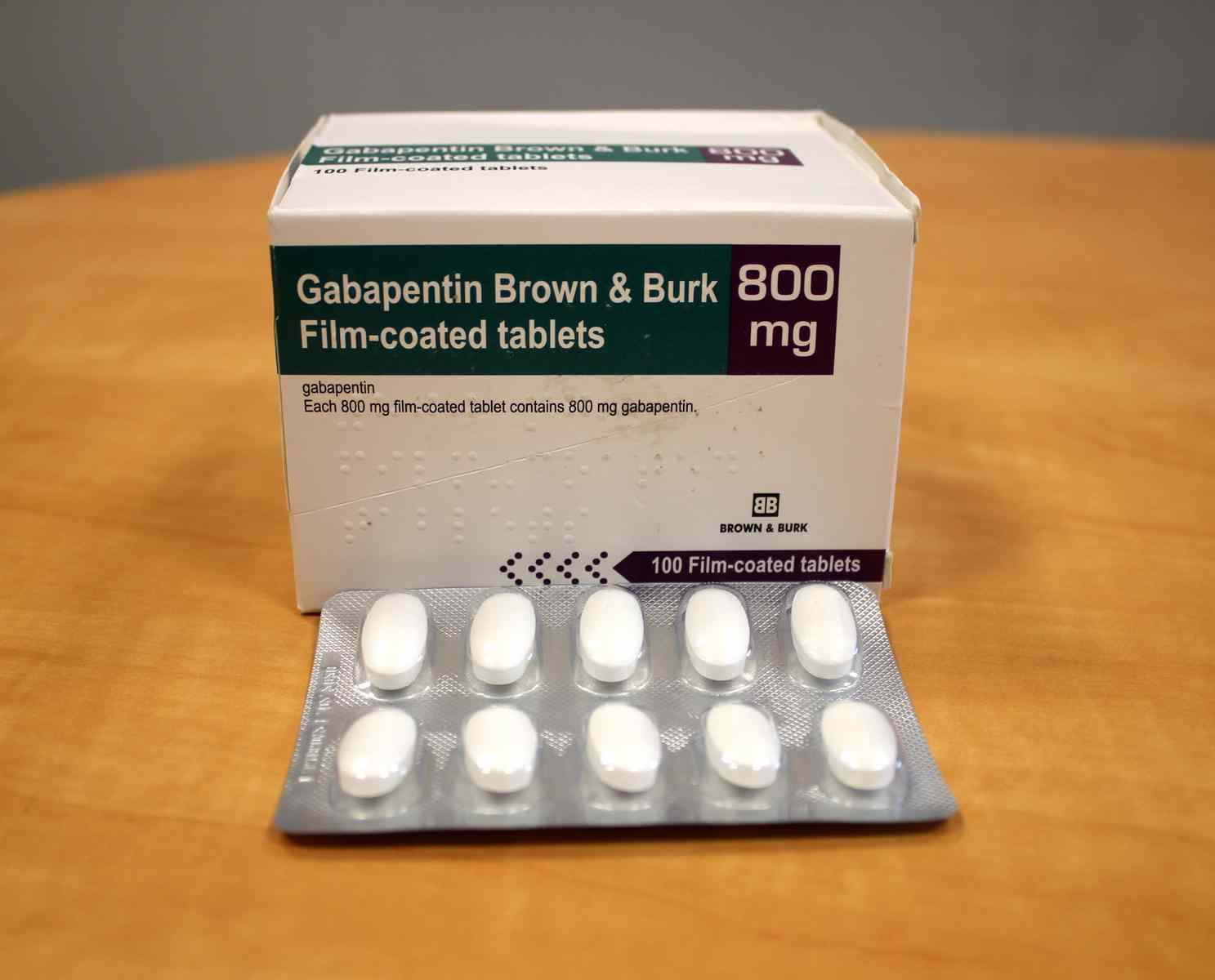
Health Canada's review concluded that there is evidence supporting a risk of serious breathing problems when gabapentin is used. Health Canada recommended updates to the product information for gabapentin to warn about this risk. Pediatrics (< 18 years of age): Based on the data submitted and reviewed by Health Canada, the safety and efficacy of gabapentin in pediatric patients has not been established; therefore, Health Canada has not authorized an indication for pediatric use. (See . 7.1.3 Pediatrics ). 1.2 Geriatrics Gabapentin is widely prescribed. Gabapentin is a commonly prescribed medication, with about 3.9 million prescriptions filled in Canada in 2015.1 Gabapentin is approved by Health Canada as adjunctive therapy in the management of epilepsy.1 However, the most common recommendations and prescribing internationally are for off-label conditions, including anxiety, alcohol use disorder and chronic pain.2 OTTAWA – Health Canada is advising Canadians about the increased risk of opioid overdose and serious side effects when taking gabapentin (e.g., Neurontin) or pregabalin (e.g., Lyrica) with an opioid. Gabapentin is authorized to treat epilepsy and pregabalin is authorized to treat nerve pain. safety and efficacy of gabapentin in pediatric patients has not been established; therefore, Health Canada has not authorized an indication for pediatric use. (See 7.1.3 Pediatrics). 1.2 Geriatrics Geriatrics (>65 years of age): No data are available to Health Canada; therefore, Health Canada has not authorized an indication for geriatric use. GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Gabapentin is approved by Health Canada as an antiepileptic agent and it is also widely used off-label in the treatment of pain and in the management of several psychiatric conditions, including alcohol withdrawal and cocaine dependence. 1-4 The exact mechanism by which this GABAPENTIN belongs to the family of medicines called antiepileptic drugs and is used for treating epilepsy (seizures). GABAPENTIN has been prescribed for you by your doctor to reduce your number of seizures. TEVA-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. 1.1 Pediatrics Pediatrics (< 18 years of age): Based on the data submitted and reviewed by Health Canada, the Health Canada's review concluded that there is evidence supporting a risk of serious breathing problems when gabapentin is used. Health Canada recommended updates to the product information for gabapentin to warn about this risk. Health Canada has issued new restrictions concerning the use of gabapentin. To read the full Health Canada Advisory, visit Health Canada's web site at healthycanadians.gc.ca . Alcohol and other medications that cause drowsiness: People taking this medication should not combine it with alcohol and avoid combining it with other medications that You may report side effects to Health Canada at 1-866-234-2345. Precautions Before taking gabapentin, tell your doctor or pharmacist if you are allergic to it; or to gabapentin enacarbil; or if you have any other allergies. Approved Drug Products containing Gabapentin listed with Health Canada. Original Data : Health Canada Website Information about the product including what the product is used for, dosage, warnings, proper use and side effects. This summary will not tell you everything about the product. Contact your healthcare professional if you have any questions about the product. Information on health products evolves as a product goes through its life-cycle. This may start at the point of clinical trials, and then continues once the product becomes available on the Canadian market. Health Canada's understanding of the risks, benefits and uncertainties of a given health product evolves the longer that the product is used. High-dose gabapentin is associated with a twofold increase in adverse efects, including somnolence, tremors, ataxia and nystagmus. Note: GABA = γ-aminobutyric acid, OR = odds ratio. Summary safety review — gabapentin — assessing the potential risk of serious breathing problems. Ottawa: Health Canada; 2016. TEVA-GABAPENTIN (gabapentin) Product Monograph Page 3 of 32 TEVA-GABAPENTIN (gabapentin) PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Dosage Form / Strength Nonmedicinal Ingredients oral Capsules 100 mg, 300 mg, and 400 mg NEURONTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. 1.1 Pediatrics Pediatrics (< 18 years of age): Based on the data submitted and reviewed by Health Canada, the We would like to show you a description here but the site won’t allow us. Gabapentin is not approved by Health Canada for use in children and adolescents.While not approved by Health Canada for these uses, there is evidence to support the use of gabapentin in adults with neuropathic pain, anxiety, restless legs syndrome, cannabis use disorder, and alcohol use disorder.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |