Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 | |
 | |
 |  |
 |
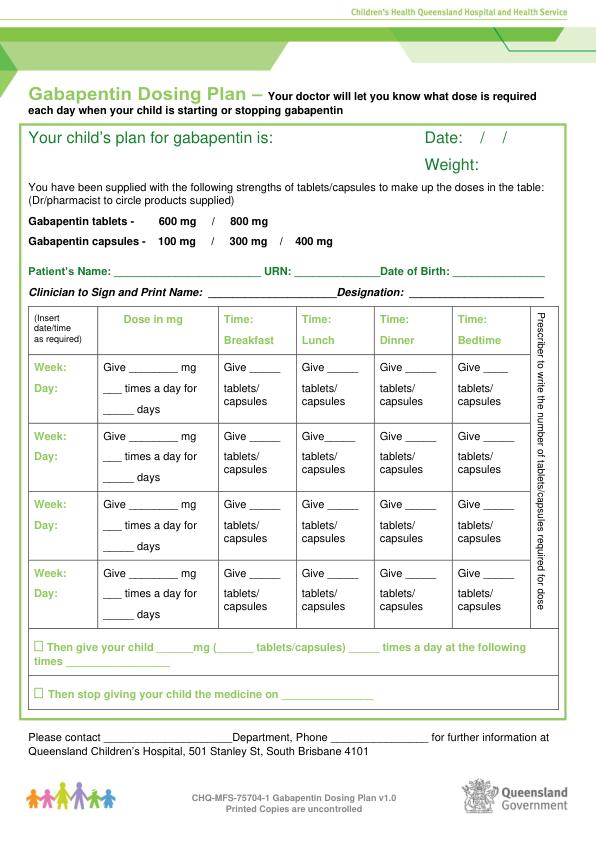
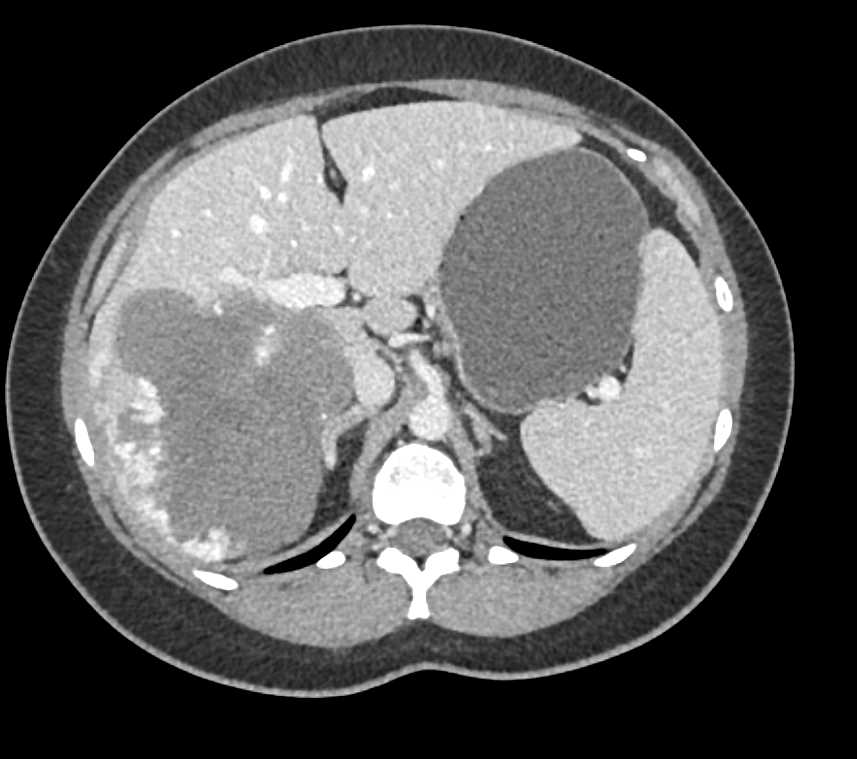
For gabapentin, unlike disulfiram and naltrexone, no need for hepatic dose adjustments is evidenced. Gabapentin can also be used in patients with renal function below 20 mg/dl (although a dosing adjustment is needed). HD: decr. dose proportionately from CrCl 15; give 125-350 mg as supplement after dialysis; PD: 300 mg q48h; supplement not defined [extended-release form] CrCl 30-60: decr. usual maint. dose to 600-1800 mg ER qd; CrCl 30: avoid use HD: avoid use; PD: not defined hepatic dosing [no adjustment] Child 6–11 years 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose maximum to be given in 3 divided For gabapentin, unlike disulfiram and naltrexone, no need for hepatic dose adjustments is evidenced. Gabapentin can also be used in patients with renal function below 20 mg/dl (although a dosing adjustment is needed). Pharmacology. Gabapentin and pregabalin are commonly used first-line agents for diabetic peripheral neuropathy and other common neuropathies. Pharmacologically, both agents inhibit alpha-2-delta (α2δ) subunit of N-type voltage-gated calcium channels, a key receptor involved in regulating the excitability of neurons. 3 Peripheral nerve injury results in the upregulation of α2δ-1 receptors For gabapentin, unlike disulfiram and naltrexone, no need for hepatic dose adjustments is evidenced. Gabapentin can also be used in patients with renal function below 20 mg/dl (although a dosing adjustment is needed). Hepatic Impairment. Because gabapentin is not metabolized, studies have not been conducted in patients with hepatic impairment. Renal Impairment. Dosage adjustment of GRALISE is necessary in patients with impaired renal function. GRALISE should not be administered in patients with creatinine clearance <30 mL/min or in patients undergoing The recommended initial dose for adults is 300 mg three times daily increasing as needed to a maximum dose of 1800 mg daily. The most common side effects of gabapentin are dose related and include dizziness, somnolence, tremor, ataxia, blurred vision, anxiety, and gastrointestinal upset or nausea. We would like to show you a description here but the site won’t allow us. Hydromorphone: Some studies report a C max increased up to four times following a single immediate-release dose of hydromorphone in patients with moderate liver disease, due to decreased first-pass metabolism in liver dysfunction and high hepatic extraction. 4 It has been recommended to start with lower doses but maintain a similar dosing Hepatic Dose : No dosage adjustments are needed unless there is concomitant renal dysfunction. Dosage adjustment in patients undergoing hemodialysis is necessary (see DOSAGE AND ADMINISTRATION). Hepatic Disease: Because gabapentin is not metabolized, no study was performed in Gabapentin and Cirrhosis of the Liver - Fatty Liver Disease • Start with low dosage and use extended dosing intervals (eg, every 6‐12 hour intervals). • Dose is based on renal function. • Avoid morphine in patients with renal disease. • Avoid NSAIDs in patients with cirrhosis. • Monitor carefully and frequently for sedation. Medscape - Seizure dosing for Neurontin, Gralise (gabapentin), frequency-based adverse effects, comprehensive interactions, contraindications, pregnancy & lactation schedules, and cost Detailed Gabapentin dosage information for adults and children. Includes dosages for Restless Legs Syndrome, Epilepsy and Postherpetic Neuralgia; plus renal, liver and dialysis adjustments. In most cases, gabapentin doesn’t hurt the liver or kidneys, though proper dosing is important to prevent side effects. Learn how gabapentin affects the liver and kidneys here. Max dosage 3600mg if patient already on gabapentin; Taper dose > 7 days to discontinue; Pediatric Dosing Partial seizures. Adjunct for partial seizures with out secondary generalization in patients> 12yo with epilepsy; also adjunctive therapy for partial seizures in patients 3-12 years <3 years: Safety and efficacy not established The starting dose is 300 mg three times a day. The recommended maintenance dose of gabapentin is 300 mg to 600 mg three times a day. Dosages up to 2400 mg/day have been administered in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration. Gabapentin is not extensively protein-bound with its bioavailability most pronounced at lower dose levels . Gabapentin has no appreciable liver metabolism, yet, suspected cases of gabapentin-induced hepatotoxicity have been reported. Per literature review, two cases of possible gabapentin-induced liver injury have been reported.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 | |
 | |
 |  |
 |