Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
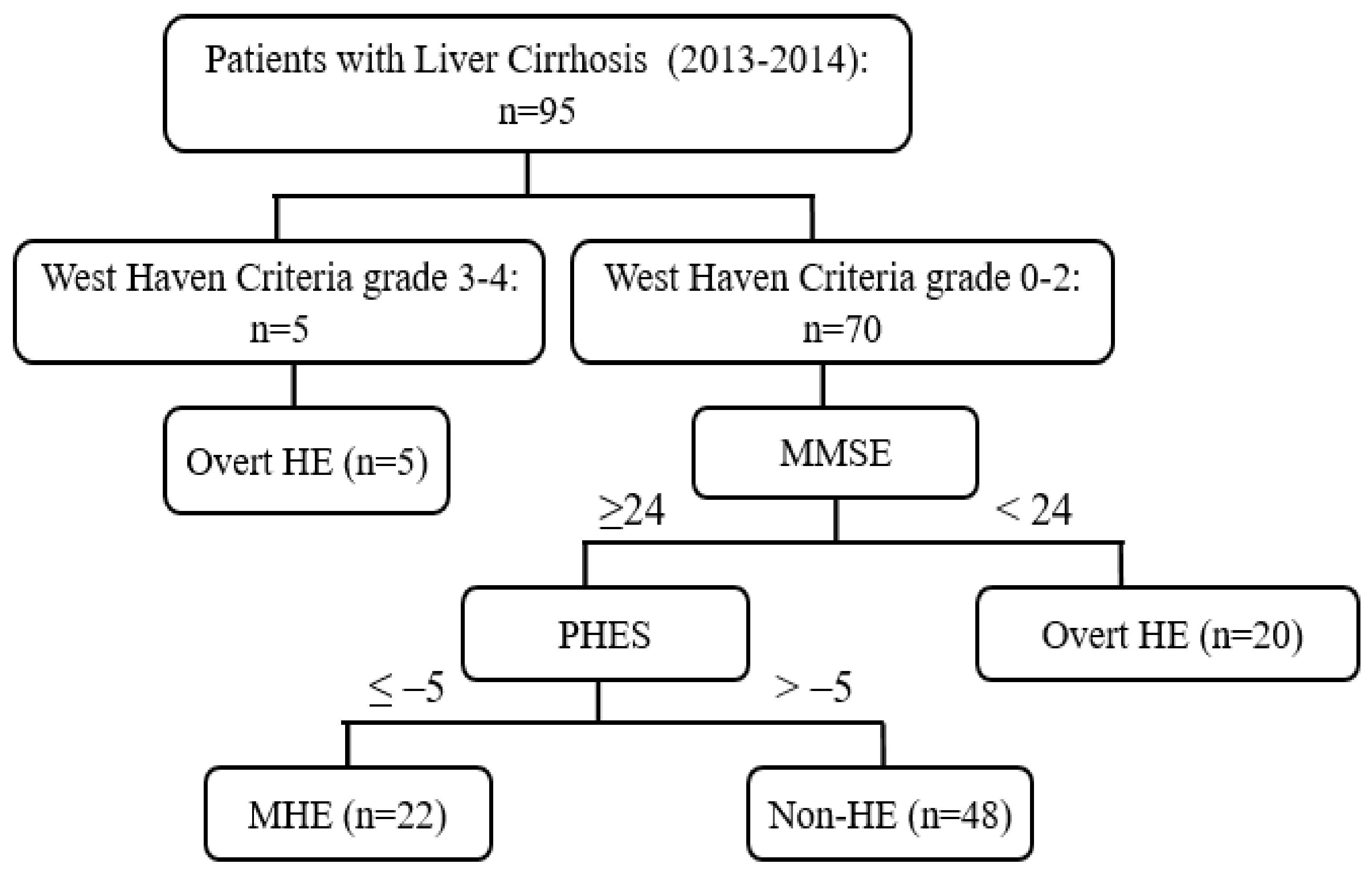 |  |
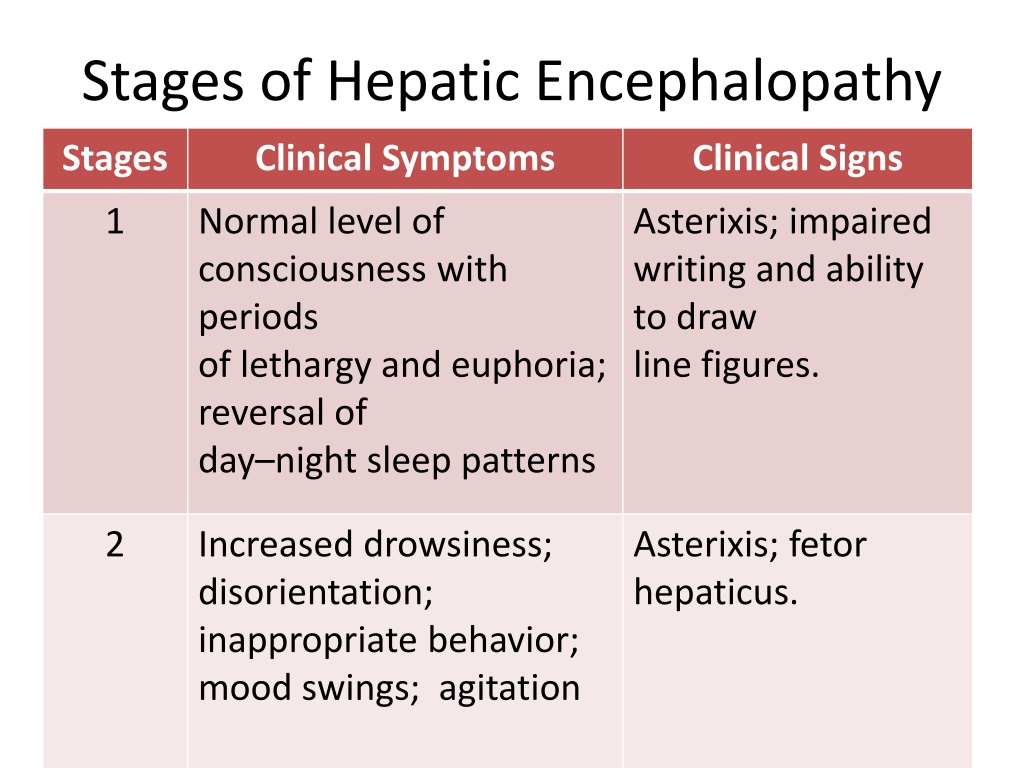
Opioids carry the risk of precipitating hepatic encephalopathy and should generally be avoided, when possible. If clinical situation demands their use, opioid use should be limited to short-acting agents for short duration. Gabapentin and pregabalin are generally safe. Duloxetine should be avoided in hepatic impairment. These findings are the bases of the following hypotheses: (i) when the liver fails, gut-derived GABA in plasma crosses an abnormally permeable blood-brain barrier and by mediating neural inhibition contributes to hepatic encephalopathy; (ii) an increased number of GABA receptors in the brain found in liver failure increases the sensitivity of th Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Deprescribing is a promising intervention to reduce the burden of hepatic encephalopathy. In fact, one single-centre study reported that an opioid prescription at discharge increased overall readmissions, but not hepatic encephalopathy-related readmissions over 6 months. 6 Furthermore, in a longitudinal cohort of United States veterans, baseline opioid use was not found to be an independent predictor of hepatic encephalopathy. 15 Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Deprescribing is In patients with cirrhosis, it is recommended that PPI use be limited to specific indications, e.g. peptic ulcer disease, because of concerns of a possible association with poorer outcomes (e.g. increased risk of spontaneous bacterial peritonitis, hepatic encephalopathy and mortality). 44,45 In cirrhosis, there is a significant increase in Gabapentin has been known to cause hyperammonaemia and toxic encephalopathy in the absence of liver failure . The paracetamol dose was low, but it is possible that it contributed through a subclinical liver inflammation, and thereby reduced liver function as seen in other UCDs [ 18 ]. Gabapentin is an uncommon cause of DILI reported to cause a hepatocellular, cholestatic, or mixed picture of liver injury. Given the limitations of prior cases, we feel our report most closely ties gabapentin use to the resultant transaminase elevation. The apparent absence or low rate of significant hepatotoxicity from gabapentin may be due to its minimal hepatic metabolism and rapid urinary excretion. Overt hepatic encephalopathy — Patients with overt hepatic encephalopathy have clinically apparent impairments in cognitive and neuromuscular function. Treatment includes determining the appropriate setting for care, correcting any predisposing conditions (particularly dehydration or infection), and medications such as lactulose. Hepatic encephalopathy (HE) is a potentially reversible neurocognitive complication of cirrhosis. It has been reported in at least 30% of patients with cirrhosis and imposes a significant economic burden on caregivers and the healthcare system. Ammonia has been recognized as the culprit in HE development, and all the currently approved treatments mostly act on this toxin to help with HE Gabapentin-induced encephalopathy. Gabapentin-induced encephalopathy. Gabapentin-induced encephalopathy Clin Neurophysiol. 2015 Apr;126(4) :845-6. Multiple medications are associated with an increased risk of incident hepatic encephalopathy. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Purpose: To date, only one case of asterixis associated with the use of gabapentin (GBT) has been reported. No data, instead, are available on the occurrence of asterixis related to a dementing encephalopathy during GBT therapy in the elderly. Hepatic encephalopathy is reported as a side effect among people who take Gabapentin (gabapentin), especially for people who are female, 60+ old, have been taking the drug for < 1 month also take Xifaxan, and have Hepatitis c. Higher risk of encephalopathy was reported in patients with history of severe hepatic encephalopathy, ethanol-related hepatic failure, metabolic liver disease, greater severity of pre-transplant liver injury (defined by Child-Pugh or MELD scores) and non-elective liver transplantation[41,46,47]. Quite often, multiple co-existing risk factors Background and aims: Multiple medications are associated with an increased risk of incident hepatic encepha-lopathy. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin/pregabalin, and/or proton pump inhibitors are increasingly prescribed to persons with cirrhosis. Deprescribing is a promising intervention to reduce the burden of hepatic encephalopathy. Background and aims Multiple medications are associated with an increased risk of incident hepatic encephalopathy. Despite this known risk, medications such as opioids, benzodiazepines, gabapentin Prescribing advise for patients with hepatic impairment. Hepatotoxicity is either dose-related or unpredictable (idiosyncratic). Drugs that cause dose-related toxicity may do so at lower doses in the presence of hepatic impairment than in individuals with normal liver function, and some drugs that produce reactions of the idiosyncratic kind do so more frequently in patients with liver disease.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |