Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
00110-0/asset/52dc3cdc-6865-49eb-827f-f6defd446616/main.assets/gr1c.gif) |  |
 |  |
 |  |
 |  |
 |
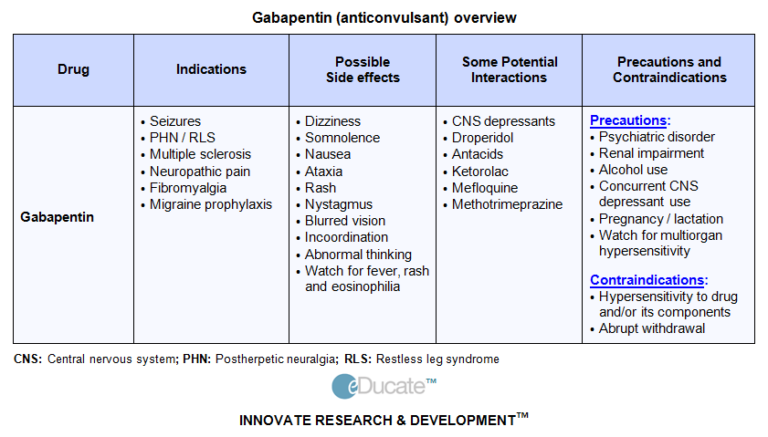
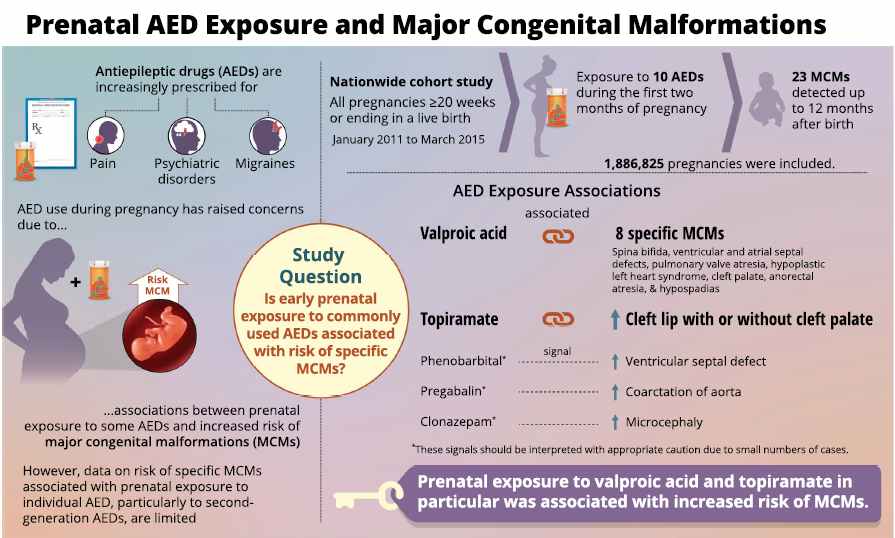
All pregnant women in the UK will be offered a very detailed anomaly scan at around 20 weeks of pregnancy as part of their routine antenatal care. No extra monitoring for major birth defects is required following gabapentin use in pregnancy. Babies exposed to gabapentin before delivery may experience withdrawal symptoms for a few days after birth. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early While gabapentin (Neurontin) is now used in a wide variety of clinical settings — for epilepsy, pain management, restless leg syndrome, anxiety, and sleep disturbance – there is relatively little information regarding its reproductive safety. Most recently, a prospective study from researchers at the Motherisk program reports on the outcomes of 223 pregnancies exposed to gabapentin Animal studies have shown that gabapentin intake during pregnancy increases the risk of embryo fetal toxicity, specifically on mice. For rats, adverse effects on offspring development were common among those who had gabapentin during pregnancy. Talk to your doctor about taking gabapentin if you are pregnant or are planning on being pregnant. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. Does taking gabapentin increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Small, controlled studies on gabapentin have not suggested an increased chance of birth defects. Does taking gabapentin increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Small, controlled studies on gabapentin have not suggested an increased chance of birth defects. Gabapentin is not generally recommended in pregnancy as there is not enough information about whether it's safe for your baby. However, from the small amount of information that is available, there's no clear evidence that it's harmful. Our objectives were to 1) determine whether first-trimester use of gabapentin is associated with an increased risk for major malformations; 2) examine rates of spontaneous abortions, therapeutic abortions, stillbirths, mean birth weight and Studies which assessed rates of miscarriage or intrauterine death following gabapentin exposure in pregnancy do not identify an increased risk of these outcomes. Conflicting results regarding a possible increased risk of preterm delivery and low birth weight have been described in a small number of studies but these data may be confounded. Five studies reported significant findings with increased risks of overall congenital anomalies, specific anomalies (nervous system, eyes, oro-facial clefs, urinary and genital system), miscarriage, stillbirth and specific neurodevelopmental outcomes after exposure to pregabalin during pregnancy. Risk Summary: There are no data on the developmental risks associated with use of this drug in pregnant women; in animal studies, developmental toxicity was observed at doses estimated to be similar or lower than those used clinically. Despite the widespread use, only sparse information is available on the safety of gabapentin during pregnancy. We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes. Methods and findings Using the United States Medicaid Analytic eXtract (MAX) dataset, we conducted a There was an increased risk of preterm birth among women exposed to gabapentin either late (RR=1.28 [CI 1.08-1.52], p < 0.01) or both early and late in pregnancy (RR=1.22 [1.09-1.36], p < 0.001). A meta-analysis by Veroniki et al 2017a also did not suggest an increased risk of overall major congenital malformation with gabapentin use during pregnancy. However, gabapentin was found to be Selected References: Blotiere PO, et al. 2020. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open 10(6). Brannon GE, Rolland PD. Anorgasmia in a patient with bipolar disorder type 1 treated with gabapentin. J Clin Psychopharmacol. 2000;20(3):379
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
00110-0/asset/52dc3cdc-6865-49eb-827f-f6defd446616/main.assets/gr1c.gif) |  |
 |  |
 |  |
 |  |
 |