Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 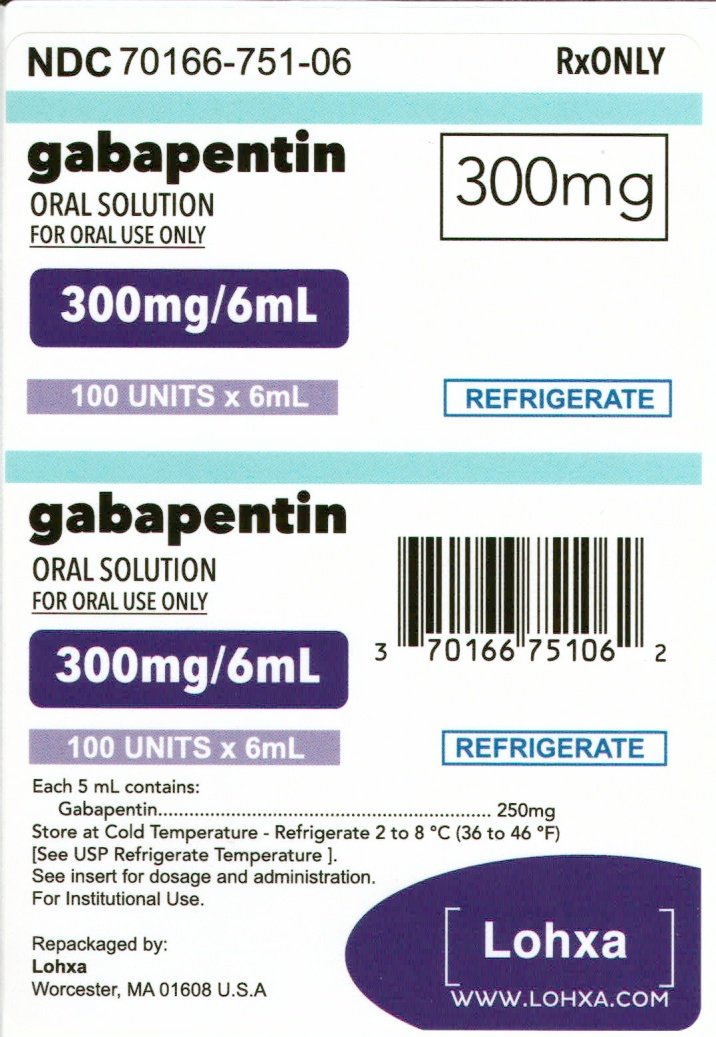 |
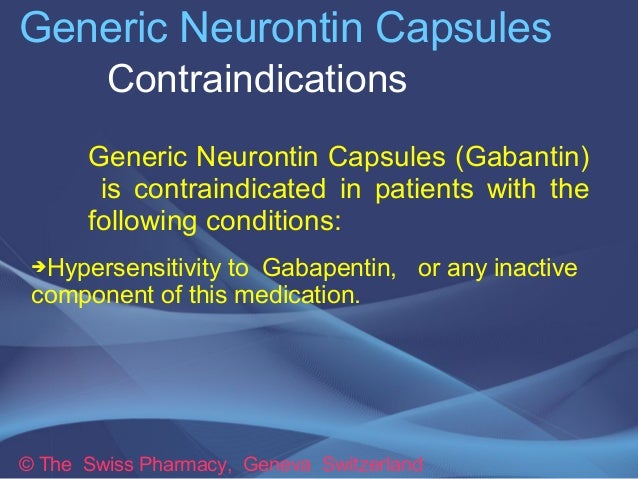
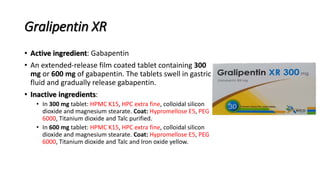
The inactive ingredients for the oral solution are glycerin, xylitol, purified water and artificial cool strawberry anise flavor. Gabapentin is described as 1-(aminomethyl)cyclohexaneacetic (gabapentin) oral solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. The 100 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. The 100 mg capsule shell contains gelatin and titanium dioxide. The 300 mg capsule shell contains gelatin, titanium dioxide, and yellow iron oxide. Inactive Ingredients in Gabapentin Formulations. While gabapentin is the star of the show, it’s essential to consider the inactive ingredients that accompany it in various formulations. These components can affect how the body absorbs the medication, how long it remains effective, and even how it tastes or feels when taken. GABAPENTIN- gabapentin solution Amneal Pharmaceuticals LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 INDICATIONS Active ingredient: gabapentin Inactive ingredients in the capsules: corn starch, D&C red 33 (300 mg only), D&C yellow 10 (300 mg only), ferric oxide red (400 mg only), ferric oxide yellow (400 mg only), ferrosoferric oxide, gelatin, lactose monohydrate, potassium hydroxide, propylene glycol, sodium lauryl sulfate, shellac, talc, and titanium of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. The 100 mg capsule shell contains gelatin and titanium dioxide. The 300 mg capsule shell contains gelatin, titanium dioxide, and yellow iron oxide. The inactive ingredients for the oral solution are glycerin, xylitol, purified water and artificial cool strawberry anise flavor. Gabapentin is described as 1-(aminomethyl)cyclohexaneacetic Brand Reference Neurontin Caps; Therapeutic Category Anticonvulsants; Pronunciation GA ba PEN tin; Inactive Ingredients mannitol, pre-gelatinized starch and talc. The 100 mg capsule shell contains titanium dioxide, gelatin and sodium lauryl sulfate. Each gabapentin tablet, USP contains 600 mg or 800 mg of gabapentin and the following inactive ingredients: copovidone, corn starch, macrogol, magnesium stearate, polyvinyl alcohol, talc and titanium dioxide. What are the ingredients in gabapentin capsules? Active ingredient: gabapentin, USP Inactive ingredients in the capsules: corn starch, magnesium stearate, mannitol and talc. The 100 mg, 300 mg and 400 mg capsule shell contains FD&C Blue No. 2, gelatin, titanium dioxide and yellow iron oxide. Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). What are the ingredients in gabapentin? Active ingredient: gabapentin. Inactive ingredients in the capsules: calcium carbonate, calcium sulfate dihydrate, glyceryl behenate, and pregelatinized maize starch. The capsule shell contains gelatin, titanium dioxide, sodium lauryl sulfate, yellow iron oxide (300 mg and 400 mg) and red iron oxide (400 mg). What excipients are in GABAPENTIN? Inactive ingredients, estimated generic entry dates, and list of branded drugs and generic equivalents. Each gabapentin capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: anhydrous lactose, cornstarch, and talc. The 100 mg capsule shell contains gelatin, sodium lauryl sulfate, and titanium dioxide. The inactive ingredients for the capsules are corn starch, gelatin, magnesium stearate, mannitol, sodium lauryl sulphate, talc, titanium dioxide, black edible ink which contains iron oxide black, potassium hydroxide, propylene glycol, and shellac. Administer NEURONTIN orally with or without food. NEURONTIN capsules should be swallowed whole with water. Inform patients that, should they divide the scored 600 mg or 800 mg NEURONTIN tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Each Neurontin capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). Each gabapentin tablet, USP intended for oral administration contains 600 mg and 800 mg of gabapentin. In addition each tablet contains following inactive ingredients: copovidone, low substituted hydroxypropyl cellulose, magnesium stearate, mannitol, poloxamer, povidone and talc. Each Gabapentin capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: Pregelatinized Maize starch, talc, gelatin, titanium dioxide, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (300 mg and 400 mg only), black iron oxide (300 mg and 400 mg only).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |