Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
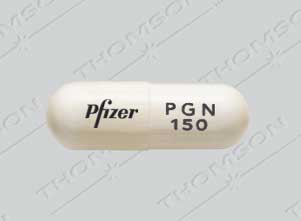
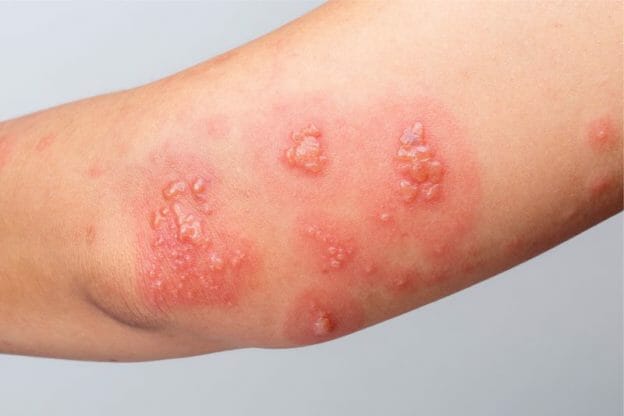
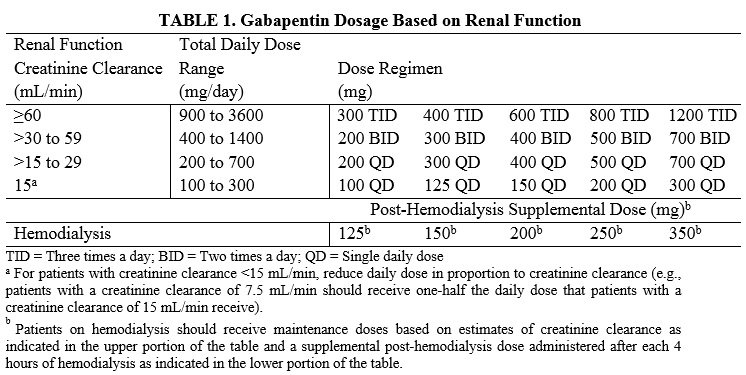
December 19, 2019. Media Inquiries Nathan Arnold 301-796-6248. The following quote is attributed to Douglas Throckmorton, M.D., deputy director for Regulatory Programs in the FDA’s Center for use of gabapentin; dosage form and strength; dose; ROA; frequency and duration of therapy; use of gabapentin in a combination product; use and formulation of gabapentin in a compounded product; use of gabapentin compared to FDA-approved drugs or other treatments; outcome measures; authors’ conclusions. HORIZANT is not the same medicine as gabapentin [for example, NEURONTIN® (gabapentin) and GRALISE® (gabapentin)]. HORIZANT should not be used in their place. Do not take these or other gabapentin products while taking HORIZANT. Before taking HORIZANT, tell your healthcare provider if you: The FDA has approved gabapentin and pregabalin for several conditions, from seizures and restless leg syndrome to diabetes and fibromyalgia. Although not specifically approved to treat chronic Gabapentin and pregabalin are FDA-approved for a variety of conditions, including seizures, nerve pain, and restless legs syndrome. Our evaluation shows that the use of these It's used off-label to treat menopausal hot flashes, back pain, nerve pain, fibromyalgia, itching, restless leg syndrome and more. While it works well for some of these conditions, Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Gabapentin has been approved by the FDA to treat two different conditions and is used off-label for the treatment of many other conditions. Brand names of gabapentin include Gralise, Horizant, and Neurontin. BACKGROUND: Gabapentinoids are FDA-approved to treat a variety of conditions including partial seizures and nerve pain from spinal cord injury, shingles, and diabetes. Other approved uses Neurontin is approved by the FDA for the treatment of the following conditions: Seizures : This medication is used to reduce the occurrence of seizures in children and adults with epilepsy. It can be taken alongside other seizure medication. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. The authors concluded that gabapentin is associated with reduction in acute pain associated with postherpetic neuralgia and peripheral diabetic neuropathy (the later indication is not approved by the FDA), and that there is limited evidence to support the use of gabapentin for other types of neuropathic pain and pain disorders. 1 This Editorial Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. Gabapentin and pregabalin are FDA-approved for a variety of conditions, including seizures, nerve pain, and restless legs syndrome. Our evaluation shows that the use of these medicines, often 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or Gabapentin is FDA-approved for the following conditions: In addition to its FDA-approved applications, gabapentin has been used and studied widely off-label in the treatment of other conditions, including migraine and other types of headaches.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |