Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 | |
 |  |
 |  |
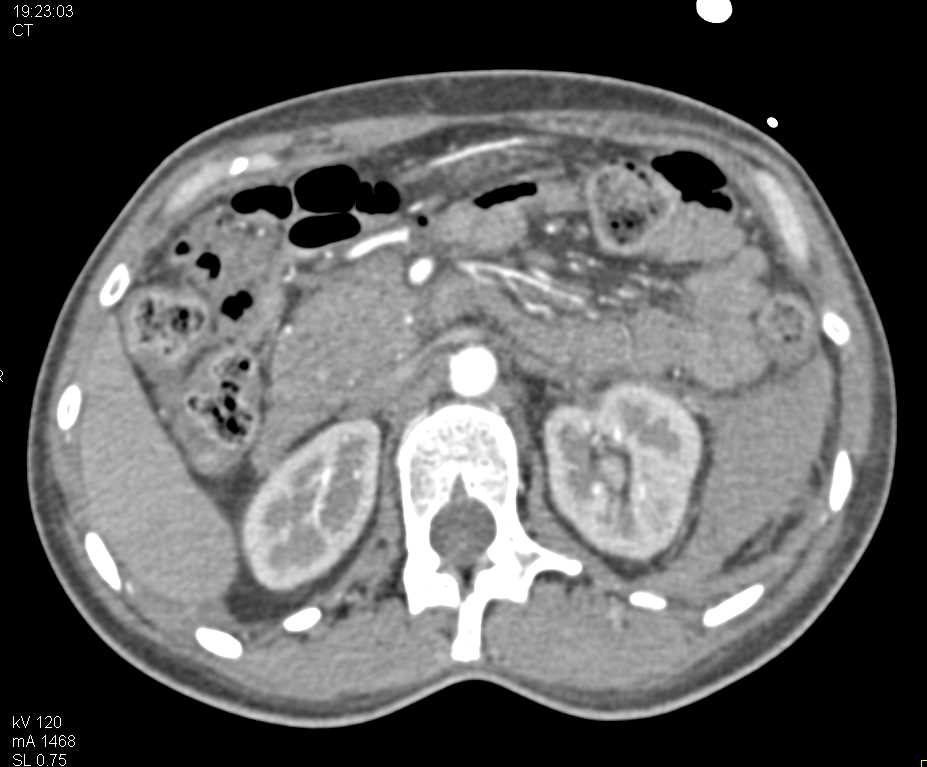
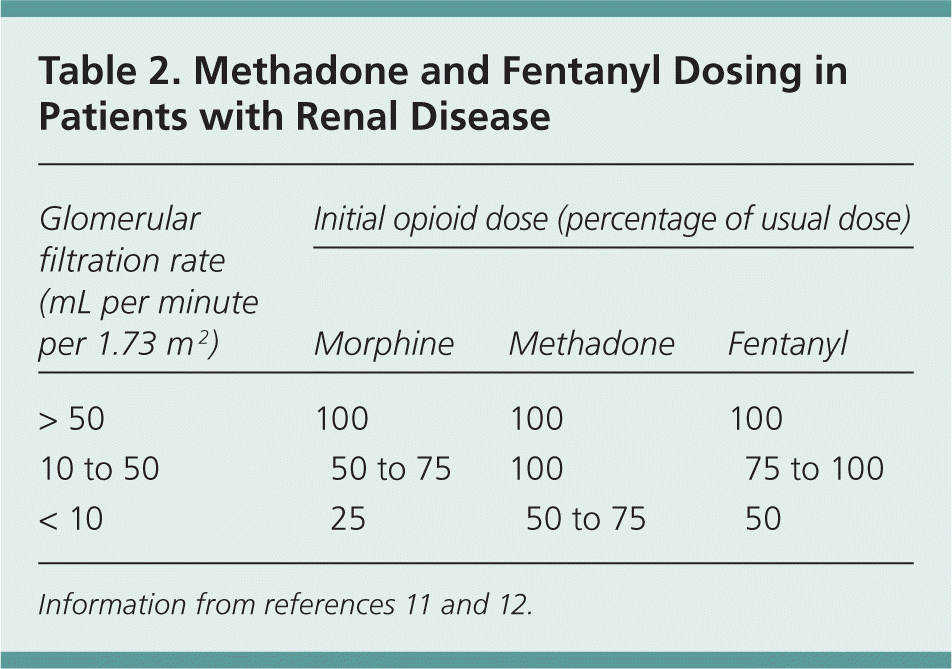
Gabapentin toxicity in patients with chronic kidney disease: A preventable cause of morbidity. The American Journal of Medicine . GoodRx Health has strict sourcing policies and relies on primary sources such as medical organizations, governmental agencies, academic institutions, and peer-reviewed scientific journals. The short answer is: yes, gabapentin can be problematic for individuals with kidney failure and chronic kidney disease (CKD). While gabapentin is often prescribed for pain management, particularly nerve pain, and sometimes for seizures, its primary elimination pathway is through the kidneys. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Myoclonus is a well-reported complication of gabapentin toxicity especially in patients with renal impairment. As gabapentin is solely excreted by the kidneys, renal dose adjustment is recommended in the literature. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. Exposure: Higher-dose gabapentinoids (gabapentin >300 mg/d or pregabalin >75 mg/d) versus lower-dose gabapentinoids (gabapentin ≤300 mg/d or pregabalin ≤75 mg/d). Outcomes: The primary composite outcome was the 30-day risk of a hospital visit with encephalopathy, a fall, or a fracture or a hospitalization with respiratory depression. Higher-dose gabapentinoids (gabapentin >300 mg/d or pregabalin >75 mg/d) versus lower-dose gabapentinoids (gabapentin ≤300 mg/d or pregabalin ≤75 mg/d). Outcomes The primary composite outcome was the 30-day risk of a hospital visit with encephalopathy, a fall, or a fracture or a hospitalization with respiratory depression. Rationale & Objective: Gabapentinoids are opioid substitutes whose elimination by the kid-neys is reduced as kidney function declines. To inform their safe prescribing in older adults with chronic kidney disease (CKD), we examined the 30-day risk of serious adverse events according to the prescribed starting dose. Since gabapentin is cleared solely by renal excretion, dosing requires consideration of the patient's renal function. Myoclonic activity may occur as a complication of gabapentin toxicity, especially with acute kidney injury or end-stage renal disease. patients with decreased kidney function (quality of the evidence grade as A and B, respectively). Limited clinical data were available (24 patients with gabapentin toxicity and 7 with pregabalin toxicity received ECTR). Severe toxicity, mortality, and sequelae were rare in cases receiving ECTR and in historical controls receiving standard care Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Discussion: Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its well recieved pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. toxic manifestations. Gabapentin toxicity was suspected initially in only 41.5% of symptomatic cases. CONCLUSION: Gabapentin toxicity in patients with chronic kidney disease is underrecognized. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, Gabapentinoids are opioid substitutes whose elimination by the kidneys is reduced as kidney function declines. To inform their safe prescribing in older adults with chronic kidney disease (CKD), we examined the 30-day risk of serious adverse events according to the prescribed starting dose. The range of Gabapentin toxicity across the spectrum of renal insufficiency is underrecognized. In this case report, the patient with AKI only was confused and the patient with ESRD had myoclonus and seizure in addition to confusion. There seems to be a graded increase in toxicity with the corresponding deterioration of renal function. The half-life of gabapentin immediate-release formulation is 5–7 hours in patients with normal renal function and is prolonged up to 52 hours in patients with CrCl<30 mL/min. 26 The half-life of pregabalin is 16.7 hours in patients with CrCl 30–59 mL/min, 25 hours in patients with CrCl 15–29 mL/min, and 48.7 hours in patients with CrCl<15 In most cases, gabapentin doesn’t hurt the liver or kidneys, though proper dosing is important to prevent side effects. Learn how gabapentin affects the liver and kidneys here.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 | |
 |  |
 |  |