Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 | |
 |  |
 |  |
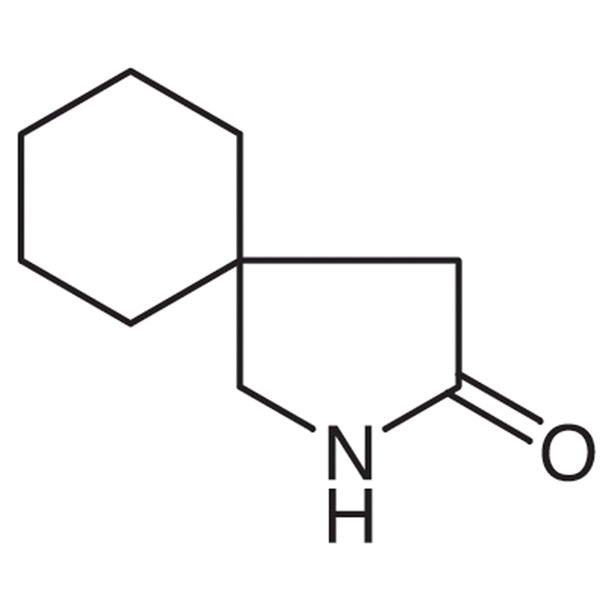
A novel process has been described on 100 g scale for the preparation of gabapentin lactam which is a penultimate intermediate for the preparation of gabapentin, comprising a Hofmann reaction of 1,1-cyclohexanediacetic acid monoamide using chlorinating agents such as trichloroisocyanuric acid, sodium dichloroisocyanurate, 1,3-dichloro-5,5-dimethylhydantoin, and N-chlorosuccinimide, which have Gabapentin is degraded directly into a high toxicity form known as gabapentin lactam (gaba-L) with a maximizing desire in mild pH and low humidity. This study reports the lactamization process of gabapentin, along with a detailed analysis of the energy landscape, geometry, and thermodynamic and kinetic preference of the process. Gabapentin is known to undergo intramolecular cyclization to form a lactam (gaba-l) with concomitant loss of water. Gabapentin was milled in a planetary mill for 15–60 min. Unmilled and milled gabapentin were stored at 50°C with relative humidity ranged between 5% and 90%. Gabapentin is degraded directly into a high toxicity form known as gabapentin lactam (gaba-L) with a maximizing desire in mild pH and low humidity. This study reports the lactamization process of gabapentin, along with a detailed analysis of the energy landscape, geometry, and thermodynamic and kinetic preference of the process. The cyclic GABA analogue gabapentin (GBP), which recently has been marketed for treatment of epilepsy, is particularly effective against complex-partial seizures as occurring in temporal lobe epilepsy. In the present study, we compared the effects of GBP and its lactam analogue (GBP-L) in the amygda Although GBP is very soluble in water and well absorbed, it is susceptible to degradation into an impurity known as gabapentin-lactam (GBP-L) via intramolecular cyclization [6]. This GBP-L product has been found to cause a higher toxicity than GBP [7]. Gabapentin-lactam (GBP-L) is a transformation product (TP) of gabapentin (GBP), a widely used anti-epileptic pharmaceutical. Due to its high persistence, GBP-L has been frequently detected in the surface water. However, the effects of GBP-L on aquatic organisms have not been thoroughly investigated. Gabapentin degrades to a cyclic lactam via an intramolecular cyclization reaction triggered by a nucleophilic attack of the hydroxyl group by the nitrogen of the amino group, followed by a dehydration reaction (Scheme 1). This degradation reaction is pH-dependent. Purpose: Gabapentin is degraded directly into a high toxicity form known as gabapentin lactam (gaba-L) with a maximizing desire in mild pH and low humidity. This study reports the lactamization process of gabapentin, along with a detailed analysis of the energy landscape, geometry, and thermodynamic and kinetic preference of the process. Gabapentin Lactam reduces protein aggregates and improves motor performance in a transgenic mouse model of Huntington disease. It opens mitochondrial ATP-dependent potassium channels. These Secondary Standards are qualified as Certified Reference Materials. There is evidence, however, that the neuroprotective properties attributed to GBP are rather associated with a derivative of GBP, gabapentin-lactam (GBP-L), which opens mitochondrial ATP-dependent K+ channels, in contrast to GBP. Gabapentin degrades directly to gabapentin-lactam (gaba-L) in the solid state. The objective of this study was to formulate a drug degradation model that accounted for the environmental storage conditions and mechanical stress (prior to storage) on lactamization kinetics. GAB can undergo lactamization and turn into a toxic form called gabapentin-lactam (LAC, Fig. 1) [38, 39], with reduced activity [40]. The rate of lactamization in aqueous solution is affected by A novel process has been described on 100 g scale for the preparation of gabapentin lactam which is a penultimate intermediate for the preparation of gabapentin, comprising a Hofmann reaction of 1,1-cyclohexanediacetic acid monoamide using chlorinating agents such as trichloroisocyanuric acid, sodium dichloroisocyanurate, 1,3-dichloro-5,5 Purpose: Gabapentin is degraded directly into a high toxicity form known as gabapentin lactam (gaba-L) with a maximizing desire in mild pH and low humidity. This study reports the lactamization The cyclic GABA analogue gabapentin (GBP), which recently has been marketed for treatment of epilepsy, is particularly effective against complex-partial seizures as occurring in temporal lobe epilepsy. In the present study, we compared the effects of GBP and its lactam analogue (GBP-L) in the amygdala kindling model of temporal lobe epilepsy. In fully kindled rats, GBP (50 mg/kg and 100 mg/kg Gabapentin is known to undergo intramolecular cyclization to form a lactam (gaba-l) with concomitant loss of water. Gabapentin was milled in a planetary mill for 15–60 min. Unmilled and milled gabapentin were stored at 50°C with relative humidity ranged between 5% and 90%. The unmilled and milled samples were assayed for gabapentin and gaba-l by reversed phase-high-performance liquid Gabapentin-lactam, a close analogue of the anticonvulsant gabapentin, exerts convulsant activity in amygdala kindled rats Naunyn-Schmiedeberg’s Arch Pharmacol (2000) 361:200–205 Digital Object Identifier (DOI) 10.1007/s002109900174 Received: 1 September 1999 / Accepted: 14 October 1999 / Published online: 3 December 1999 ORIGINAL ARTICLE Gabapentin-lactam enhanced the formation of dendritic filopodia, which are necessary for synapse formation. It also induced a network of F-actin-containing neurites. In studies with time lapse Chemsrc provides Gabapentin-lactam(CAS#:64744-50-9) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc. Articles of Gabapentin-lactam are included as well.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 | |
 |  |
 |  |