Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
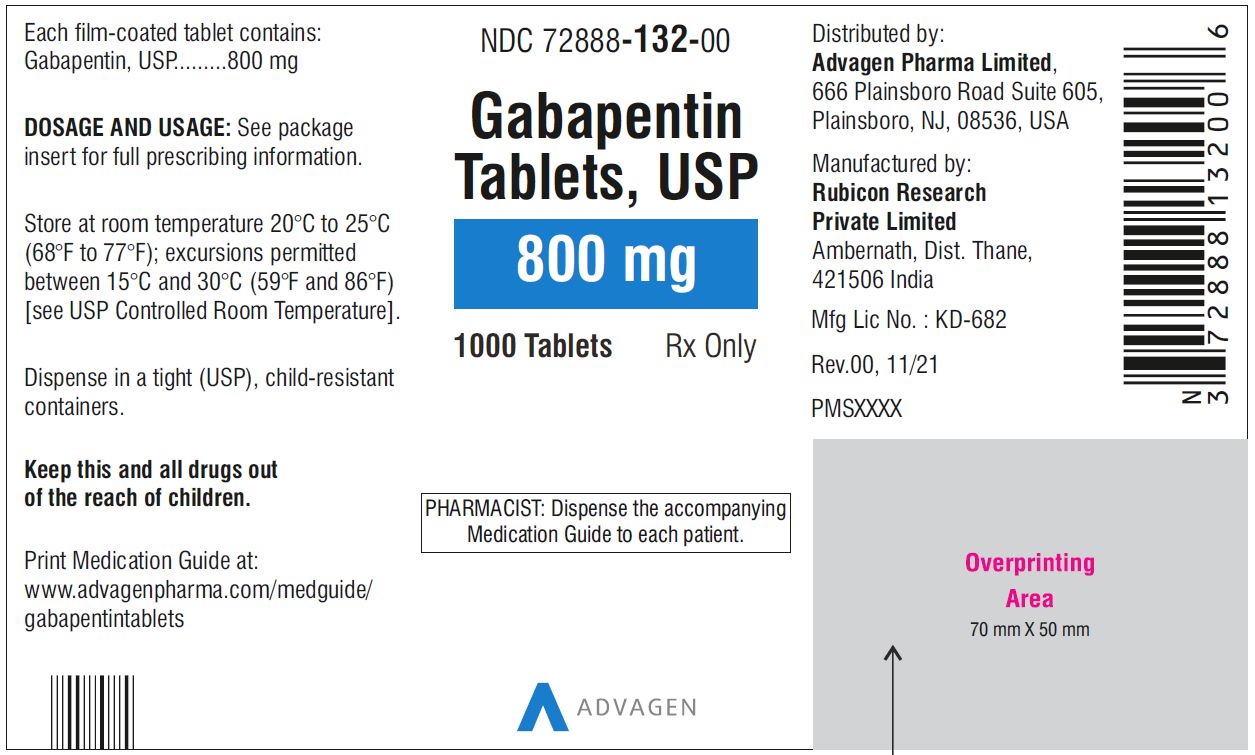
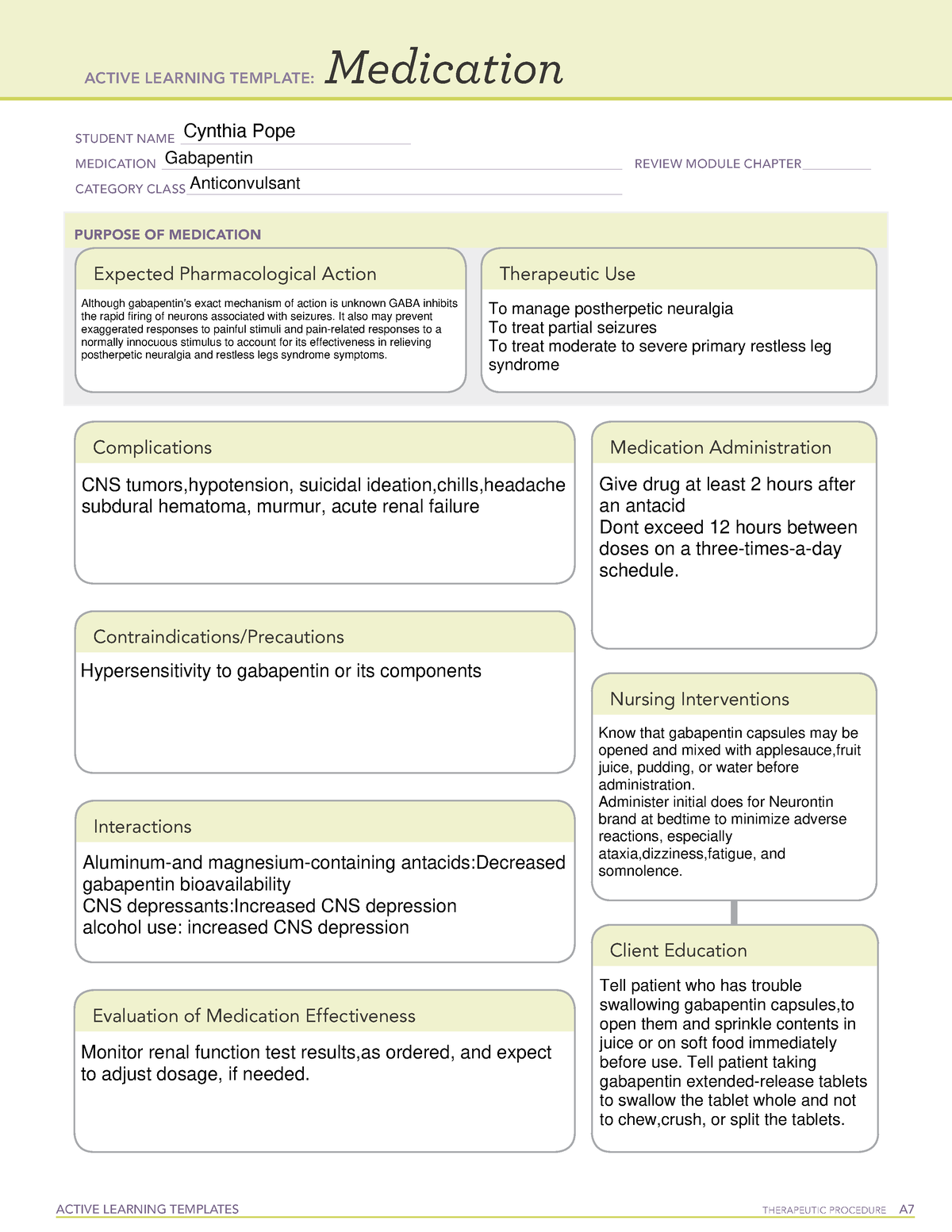
Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Gabapentin (International) In the US, Gabapentin (gabapentin systemic) is a member of the drug class gamma-aminobutyric acid analogs and is used to treat Alcohol Use Disorder, Alcohol Withdrawal, Anxiety, Back Pain, Benign Essential Tremor, Bipolar Disorder, Burning Mouth Syndrome, Carpal Tunnel Syndrome, Chronic Kidney Disease-Associated Pruritus, Chronic Pain, Cluster-Tic Syndrome, Cough Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The Misuse of Drugs Act 1971 (Amendment) Order 2018 classifies pregabalin and gabapentin as Class C drugs under paragraph 1 (b) of Part 3 of Schedule 2 to the Misuse of Drugs Act 1971 (“the 1971 Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. The law change means that it will be illegal to possess Gabapentin and pregabalin without a prescription and to supply or sell it to others. What are gabapentin and pregabalin? Gabapentin and pregabalin are drugs used to treat pain. They come in multiple forms including tablet, oral solution and capsules. Find out more about managing pain in MS Explore the classification of Gabapentin in Indiana and understand its legal implications, including possession, distribution, and prescription regulations. Gabapentin, a medication initially developed to treat seizures and nerve pain, has seen its use expand significantly in recent years. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Explore the classification of Gabapentin in North Carolina and understand its legal implications and recent legislative updates. Gabapentin, a medication primarily used to treat nerve pain and seizures, has attracted attention in North Carolina due to its misuse potential. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). The legal status of gabapentin varies significantly across different states. Some have classified it as a controlled substance while others have not. Below is an overview of states that have enacted laws regarding gabapentin: As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin Legal Implications of Gabapentin’s Status. The classification of gabapentin as a Schedule V substance in Virginia carries significant legal implications for healthcare providers, pharmacists, and patients. By placing gabapentin under this schedule, the state mandates that it be dispensed only with a prescription. Among cases classified as single substance pharmaceutical exposures (i.e., the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 6,955 cases in 2022. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |