Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
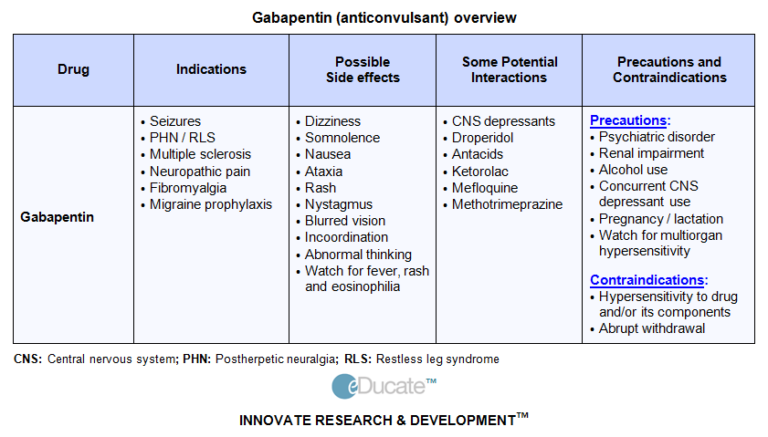
Clinical indications for gabapentin and pregabalin The drugs are licensed in the UK for treating focal seizures and managing neuropathic pain; pregabalin is licensed for treating generalised anxiety disorder. In Canada and the US, the drugs are licensed for treating pain associated with Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. Gabapentinoids are not approved for treatment of fibromyalgia in Australia, but are commonly used. 24 A 2017 systematic review of gabapentin for fibromyalgia pain found only one trial that compared gabapentin with placebo for self-reported pain, and there was no good evidence to support its use. 24 A 2022 meta-analysis of 44 RCTs reported that Dispensing gabapentin within an appropriately licensed narcotic maintenance program, such as a methadone treatment program or substance abuse treatment program. Gabapentin is used for the treatment of menopausal symptoms, A but is not licensed for this indication. In adults: Gabapentin is used for oscillopsia in multiple sclerosis, E but is not licensed for this indication. In adults: Gabapentin is used for spasticity in multiple sclerosis, E but is not licensed for this indication. Gabapentin and pregabalin (gabapentinoids) are approved in the UK and European Union for epilepsy and neuropathic pain. Pregabalin is also approved for generalised anxiety disorder. Gabapentinoid prescribing has risen dramatically, in the UK and globally, 1–4 despite limited evidence supporting their effectiveness outside the licensed MHRA and European reviews of a Nordic population-based cohort study have concluded that pregabalin use for all authorised indications during the first trimester of pregnancy may cause a slightly increased risk of major congenital malformations in the unborn child, when compared with patients taking lamotrigine, duloxetine or no antiepileptic drug. Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) 1 INDICATIONS AND USAGE . NEURONTIN. is indicated for: • Management of postherpetic neuralgia in adults • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Currently, both gabapentin and pregabalin have United States Food and Drug Administration (FDA) indications for the treatment of post herpetic neuralgia in adults and as adjunctive therapies for the management of partial-onset seizures, however pregabalin is licensed for neuropathic pain associated with diabetic peripheral neuropathy Gabapentin and pregabalin should usually be prescribed for their licensed indications (epilepsy and neuropathic pain), and generalised anxiety disorder (only pregabalin). Gabapentin is licensed for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and postherpetic neuralgia in adults [ABPI, 2020a]. Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. in 2004 while gabapentin was first authorised in the UK in 1997. Both drugs were originally licensed for seizure dis orders but now have multiple licensed indications. In England during 2016, over 12 million prescription items were dis pensed for gabapentinoids as a group.1 The Advisory Council on the Misuse of Drugs (ACMD) reported that Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. GRALISE is indicated for the management of Postherpetic Neuralgia (PHN). GRALISE should be titrated to an 1800 mg dose taken orally, once-daily, with the evening meal. GRALISE tablets gabapentin (licensed for use in peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia only) pregabalin (licensed for use in In the UK, gabapentin and pregabalin are licensed for epilepsy and neuropathic pain, and pregabalin also for generalised anxiety disorder 7.They have been recommended as first-line treatments for neuropathic pain since 2013, although evidence of efficacy is based largely on trials in post-herpetic neuralgia and painful diabetic neuropathy 8, 9. Step 1: Gabapentin 300mg once daily on day 1. Step 2: Gabapentin 300mg twice daily on day 2. Step 3: Gabapentin 300mg three times daily on day 3. Slower titration of gabapentin may be appropriate for individual patients to improve tolerability. Once a patient is on a 900mg dose, the dose can be increased in 300mg increments every two to Gabapentin tablets are indicated for: In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |