Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
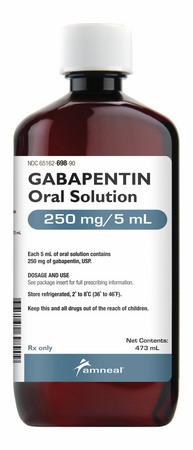
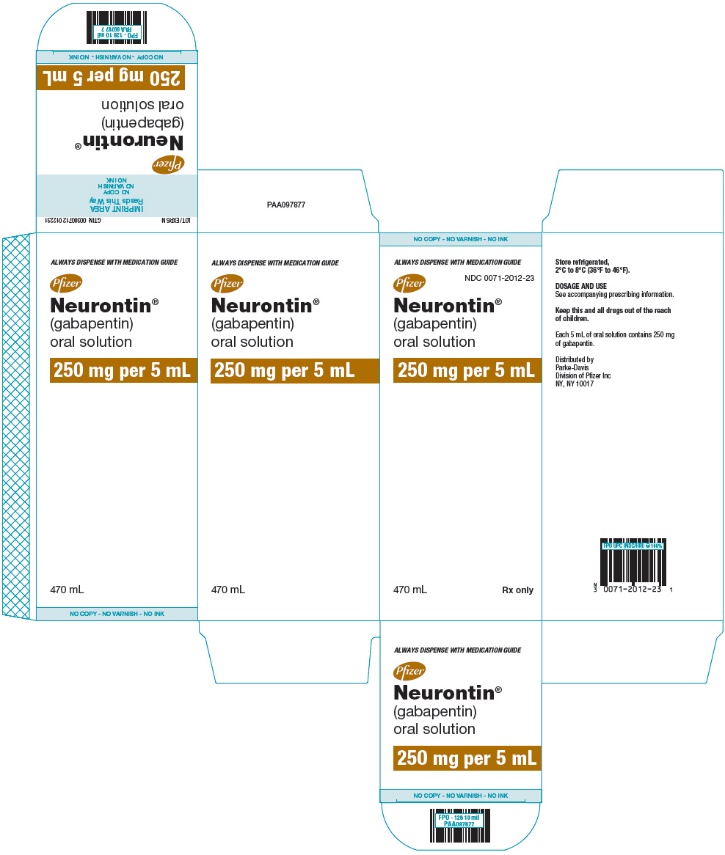
Gabapentin (2) Pregabalin (2) Specialty (1) Anaesthesia and pain (1) Switching between gabapentin and pregabalin for neuropathic pain. 20 January 2023 · An overview These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 INDICATIONS AND USAGE Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults (1) Gabapentin Oral Solution Dosage and Administration. In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Gabapentin Glenmark 50 mg/ml Oral Solution - Summary of Product Characteristics (SmPC) by Glenmark Pharmaceuticals Europe Ltd Brand Name: Neurontin. Available in 100 mg, 300 mg, and 400 mg capsules; 600 mg and 800 mg tablets; and oral solution (some products not appropriate for dogs) Background. Gabapentin was originally approved to treat epilepsy in humans. However, gabapentin became more useful as a drug to control nerve pain. Child 6–11 years 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose maximum to be given in 3 divided Therapeutic Indications: Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above. Gabapentin is also indicated for treatment of peripheral neuropathic pain such as painful diabetic neuropathy and post-herpetic neuralgia in adults. Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. (gabapentin) Oral Solution DESCRIPTION Neurontin ® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution Each ml of oral solution contains 50mg gabapentin. Excipients with known effect: Each ml of oral solution contains 1.2mg methyl parahydroxybenzoate (E218), 0.12mg ethyl parahydroxybenzoate (E214), 17.2mg propylene glycol (E1520) and 0.949mg sodium. (gabapentin) Oral Solution DESCRIPTION Neurontin ® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution Qualitative and quantitative composition. Each ml of oral solution contains 50 mg of gabapentin. For the full list of excipients, see section 6.1. 3. Pharmaceutical form. 4. Clinical particulars. Gabapentin is not bound to plasma proteins and has a volume of distribution equal to 57.7 litres. In patients with epilepsy, gabapentin concentrations in cerebrospinal fluid (CSF) are approximately 20% of corresponding steady-state trough plasma concentrations. Gabapentin is present in the breast milk of breast-feeding women. Biotransformation Gabapentin Oral Solution is a prescription medicine used to treat: z Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. Gabapentin Oral Solution is suitable for use with the following type of NG and PEG tubes: Stopping gabapentin oral solution suddenly can cause serious problems. Gabapentin oral solution can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin oral solution may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above (see section 5.1). Gabapentin is not bound to plasma proteins and has a volume of distribution equal to 57.7 litres. In patients with epilepsy, gabapentin concentrations in cerebrospinal fluid (CSF) are approximately 20% of corresponding steady-state trough plasma concentrations. Gabapentin is present in the breast milk of breast-feeding women. Biotransformation NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Gabapentin Teva is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above (see section 5.1).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |