Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
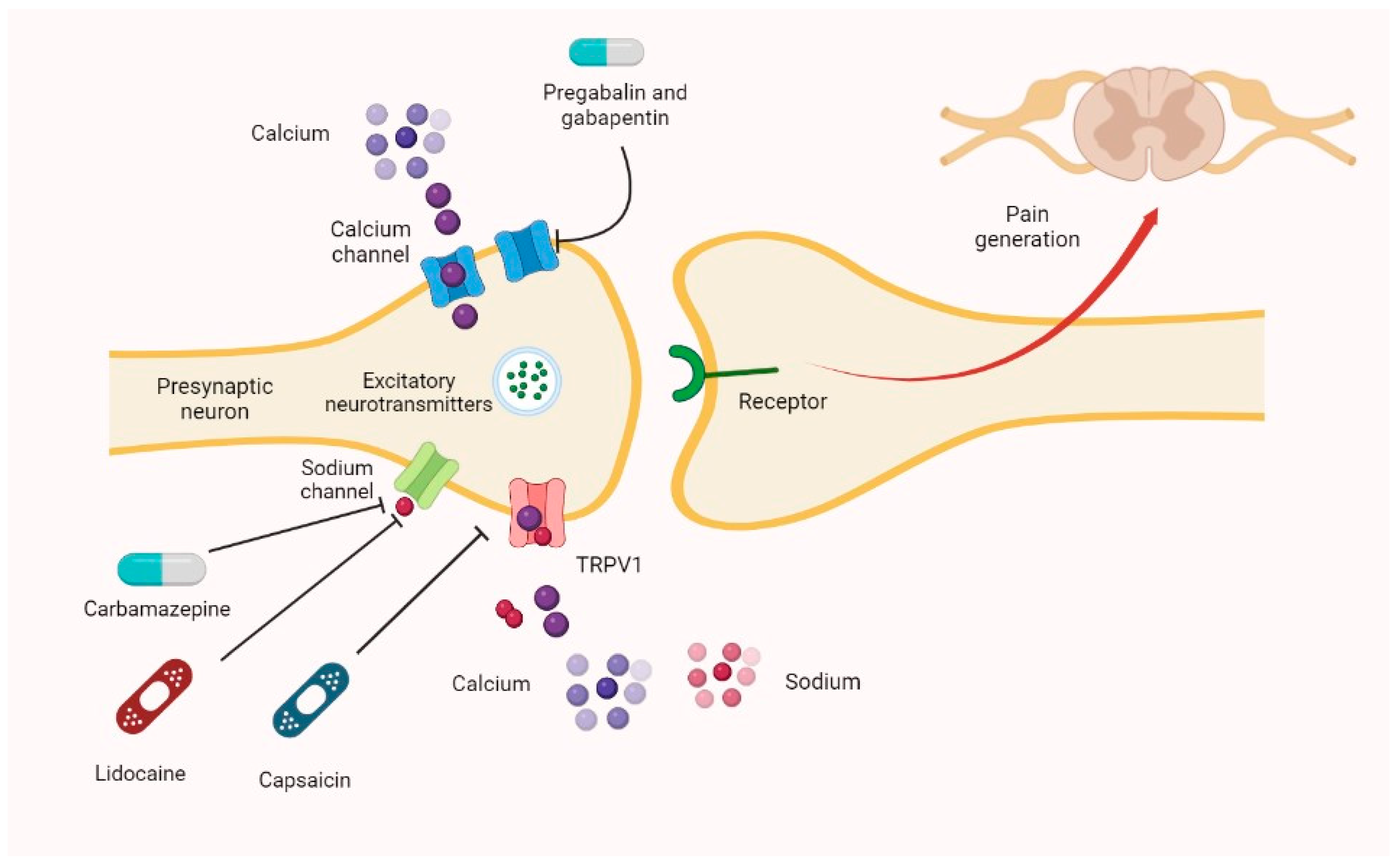 |  |
Gabapentin is commonly used to treat neuropathic pain (pain due to nerve damage). This review updates a review published in 2014, and previous reviews published in 2011, 2005 and 2000. To assess the analgesic efficacy and adverse effects of Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. cornerstone of pharmacological management of neuropathic pain.1 Despite the widespread use in neuropathic pain, the precise mechanism of action is uncertain. The effect of gaba-pentinoids in pain are assumed to be because of direct inhibi-tion of voltage gated Ca2þ channels by binding to its a2d-1 Gabapentin (GBP) is a 3,3-disubstituted derivative of gamma-aminobutyric acid (GABA). It is recommended as a first-line treatment for chronic neuropathic pain, particularly in diabetic neuropathy Mechanism of Action. Gabapentin's exact mechanism of action is not fully understood, but it is believed to work by reducing abnormal electrical activity in the brain. It is thought to bind to calcium channels, modulating their activity and reducing the release of neurotransmitters involved in seizures and nerve pain. Numerous studies confirm that gabapentinoids do not perturb normal detection and pain thresholds (Attal et al. 1998; Dirks et al. 2002); the pathophysiological state‐dependent effects of pregabalin and gabapentin implies other factors influence efficacy in neuropathic conditions. The analgesic effect in neuropathic pain is well evidenced but the role in postoperative pain is less certain. Medline and EMBASE database searches were conducted to identify studies relating to mechanisms of action and effects in experimental animal models of inflammatory and postoperative pain and human models of experimental pain. Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific binding to this subunit is described to produce Its mechanisms of action appear to be a complex synergy between increased GABA synthesis, non-NMDA receptor antagonism and binding to the α, δ subunit of voltage dependent calcium channels. The latter action inhibits the release of excitatory neurotransmitters. Gabapentinoids can be effective in some patients with neuropathic pain but more than half of the patients fail to get worthwhile pain relief. Their efficacy in non-neuropathic pain is even less impressive. Although pregabalin has more favourable pharmacokinetics as compared to gabapentin, there is little evidence to support its preferential use. The focus of perioperative pain management should be to attempt to minimise the nociceptive input and reduce the risk of transition to central sensitisation. Gabapentinoids are being increasingly used as adjuncts for management of perioperative pain. Although gabapentinoids are classed as calcium channel blockers, their mechanisms of action are poorly understood. The analgesic effect in Understanding how gabapentin works for pain is crucial for those exploring treatment options for conditions like neuropathic pain, fibromyalgia, and even post-surgical discomfort. The Mechanism of Action. Gabapentin's primary mechanism revolves around its interaction with calcium channels in the nervous system. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. Gabapentin or an α2δ-1 C terminus-interfering peptide normalizes NMDAR synaptic targeting and activity increased by nerve injury. Thus, α2δ-1 is an NMDAR-interacting protein that increases NMDAR synaptic delivery in neuropathic pain. Gabapentinoids reduce neuropathic pain by inhibiting forward trafficking of α2δ-1-NMDAR complexes. A recent study has shown the effectiveness of gabapentin (5 or 50 mg/kg, i.p.) in attenuating neuropathic pain behavior in forelimb neuropathic pain model (due to partial injury to medial and ulner nerves) in a dose-dependent manner (Yi et al. 2011). Gabapentin has become popular as a first-line treatment for neuropathic pain because of its efficacy as an antineuropathic agent and relatively benign side-effect profile. However, its mechanism of action is far from clear. The first case reports of treatment of refractory neuropathic pain conditions with gabapentin were presented in 1996. 9, 10 In 1998, confirmatory evidence was published for treatment of PHN, 11 for which it received FDA approval, as well as DPN. 12 The efficacy of pregabalin was demonstrated in several trials, gaining it approval for both Beside the implication of the noradrenergic system, it has also been suggested that descending serotonergic transmission could be important for acute gabapentinoid action in a neuropathic pain context (Suzuki et al., 2005, Bee and Dickenson, 2008), and it has been shown that pain relieving action of a high dose of gabapentin in rats with spinal Neuropathic pain. Gabapentin has proved to be efficacious in the treatment of neuropathic pain and is now approved for this indication in patients over 18 years of age. Evidence for its efficacy is discussed below. Dosage and administration. Oral doses of gabapentin are administered three times a day (tds) because of its short half-life. During the same time, Backonja et al reviewed the effect of gabapentin in 165 diabetic neuropathy patients. They showed that pain reduction in the gabapentin group is greater (as measured with an 11-point Likert scale) in comparison to the placebo group.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |