Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |
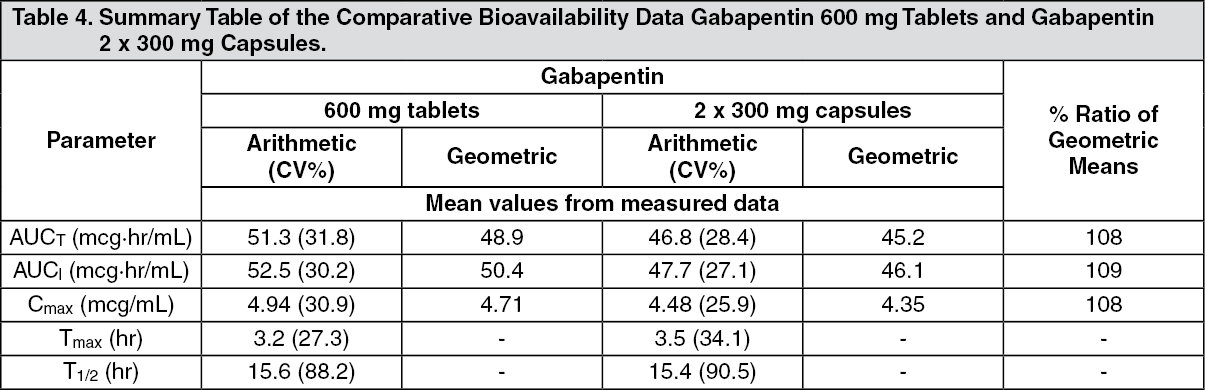
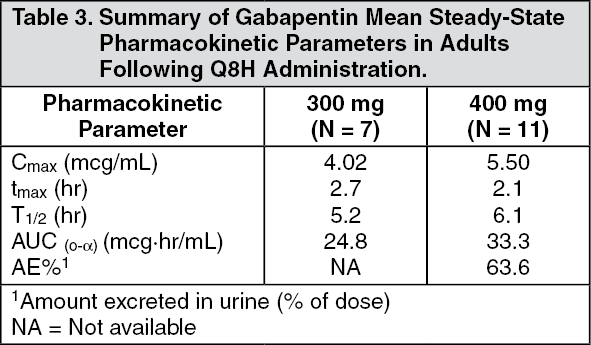
Gabapentin is a novel AED whose mechanism of action is still not fully understood. Initially designed to mimic gamma-aminobutyric acid (GABA) in the brain, gabapentin can readily pass through the blood-brain barrier. In dogs, gabapentin was useful in the treatment of epilepsy, as well as chronic, neuropathic, and post-operative pain and anxiety. In cats, it was effective in post-ovariohysterectomy-related pain and in the management of anxiety. In horses, it has been administered as an analgesic for chronic pain. Vet Clin North Am Small Anim Pract. 2013;43:1109–1125. 2. Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res. 2013;36:237–251. 3. Hurley RW, Cohen SP, Williams KA, Rowlingson AJ, Wu CL. The analgesic effects of perioperative gabapentin on postoperative pain: a meta In dogs, gabapentin was beneficial in the treatment of epilepsy, as well as chronic, neuropathic, and post-operative pain, as well as anxiety. In cats, it showed efficacy in post-ovariohysterectomy-related pain and in anxiety management. Gabapentin is usually used to manage chronic pain, especially nerve-related pain. It is also used (primarily in cats) to relieve anxiety associated with veterinary procedures, travel, and other fear-generating situations. Gabapentin can also be used as an additional medication in seizure management. Exact mechanism of action is not well understood but it is absorbed from duodenum, metabolised in the liver and excreted through kidneys. It has a half-life of 3 to 4 hours. In human medicine it is being used for treatment of seizures, different types of pain including neuropathic pain, diabetic neuropathy, malignant pain, complex regional pain Gabapentin and amantadine are used as part of analgesic protocols for chronic pain relief in dogs and cats. This article describes the types of pain, the reasons why chronic pain can be difficult to treat, and the use of gabapentin and amantadine for treatment of chronic pain. Adjuvant analgesics (ie, gabapentin, tramadol, and ketamine) are commonly used in small animal practice. Most of these drugs are prescribed for outpatients, when pain is refractory to classic analgesics (ie, local anesthetics, opioids, and nonsteroidal antiinflammatory drugs [NSAIDs]), or when contraindications exist to the administration of other analgesics, including NSAIDs. This article Robenacoxib is a non-steroidal anti-inflammatory drug used in veterinary medicine for the relief of pain and inflammation in cats and dogs. Robenacoxib: Uses, Interactions, Mechanism of Action | DrugBank Online Its mechanism of action is not entirely clear but is likely related to inhibition of calcium and, possibly, sodium channels. 1. Gabapentin is excreted unchanged in humans but is metabolized to N-methyl-gabapentin in dogs. Results in faster elimination and ability for shorter dose intervals in dogs as compared with humans 2 Greyhound dogs for gabapentin in dogs is 10-20 mg/kg [4.5 - 9.1 mg/lb] every 8 hours [10]. Plumb’s recommends gabapentin at 5-10 mg/kg [2.3 - 4.5 mg/lb] every 12 hours for chronic pain [11]. Although the bioavailability data of gabapentin comes from human research, it is possible that a similar inverse relationship Questions specific to gabapentin covered mechanisms of action, perceptions of efficacy and the potential for abuse in people. Dunn’s test for multiple comparisons and pairwise Mann–Whitney U test were used to evaluate relationships between veterinary specialty and survey responses. The mechanism of anticonvulsant action and analgesic effects is not clear, but there is evidence that the mechanism of action appears to be via blocking calcium-dependent channels. Gabapentin inhibits the alpha-2-delta (α 2 δ) subunit of the N-type voltage-dependent calcium channel on neurons. Mechanism of action. The precise mechanism through which gabapentin exerts its therapeutic effects is unclear. 16,17 The primary mode of action appears to be at the auxillary α2δ-1 subunit of voltage-gated calcium channels (though a low affinity for the α2δ-2 subunit has also been reported). 10,8,14 The major function of these subunits is Mechanism of Action. Understanding the mechanism of action of gabapentin is critical when evaluating the role that gabapentin may have as an analgesic for veterinary patients. As mentioned, gabapentin was initially intended to be a centrally acting agonist at the GABA receptor. Radulovic L L, Turck D, von Hodenberg A et al (1995) Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos 23 (4), 441-448 PubMed. Macdonald R L & Kelly K M (1993) Antiepileptic drug mechanisms of action. Epilepsia 34 (Suppl 5), S1-S8 PubMed. Other sources of information In veterinary medicine, is extra-label used in combination with other treatments to control seizures when other drugs are no longer effective or become toxic or for neuropathic pain treatment and However, there is little evidence that gabapentin is an effective analgesic for acute pain in animals. Existing evidence suggests that gabapentin may be effective as part of a multimodal analgesic treatment when combined with an opioid or an NSAID (Wagner et al. 2010; Aghighi et al. 2012; Crociolli et al. 2015). Furthermore, in the same study only half of the vets knew the exact mechanism of action of gabapentin. Gabapentin is perceived as a low-risk category for substance abuse in veterinary personnel. Although no surveys have been conducted in the UK, we believe gabapentinoids are currently prescribed very frequently worldwide. Gabapentin Gabapentin is an anticonvulsant drug, which presents an established clinical efficacy in human patients for the management of refractory partial seizures, secondarily generalized tonic-clonic seizures, and for the control of chronic neuropathic pain. Gabapentin was synthesized as a structural analogue of the inhibitory neurotransmitter GABA, with GABA-mimetic effects, able to cross the blood
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |