Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
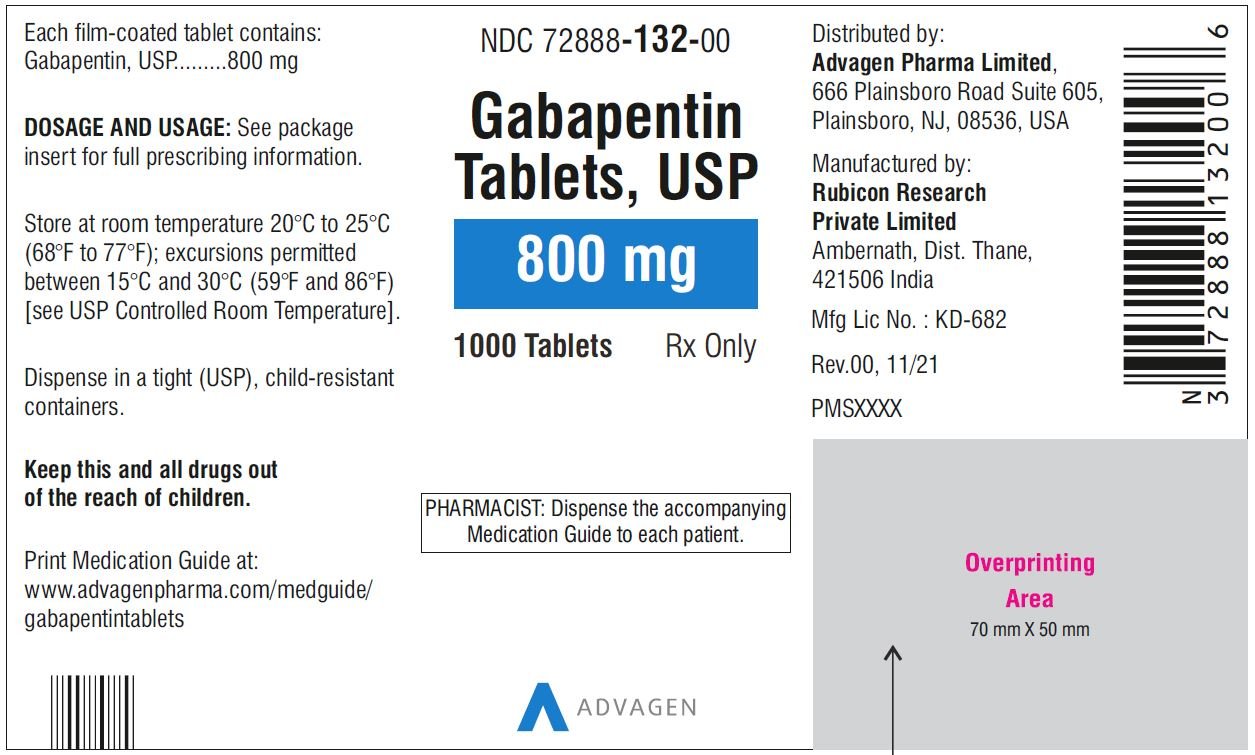 |  |
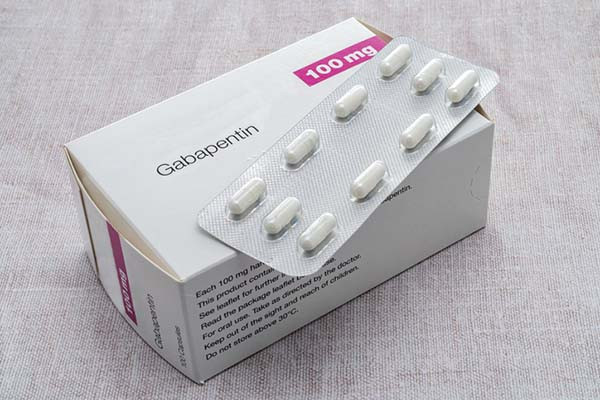
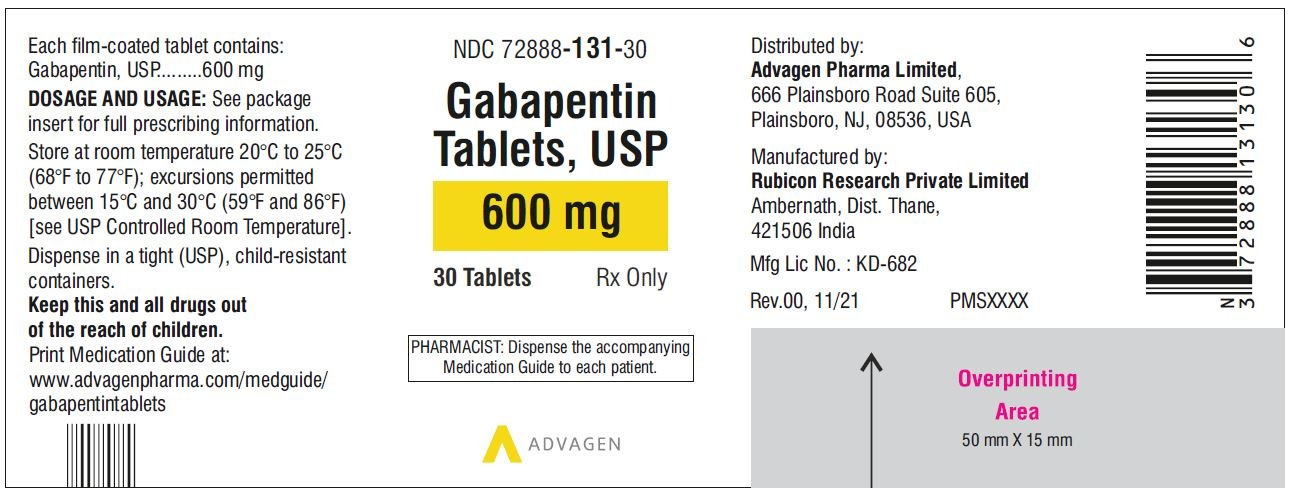
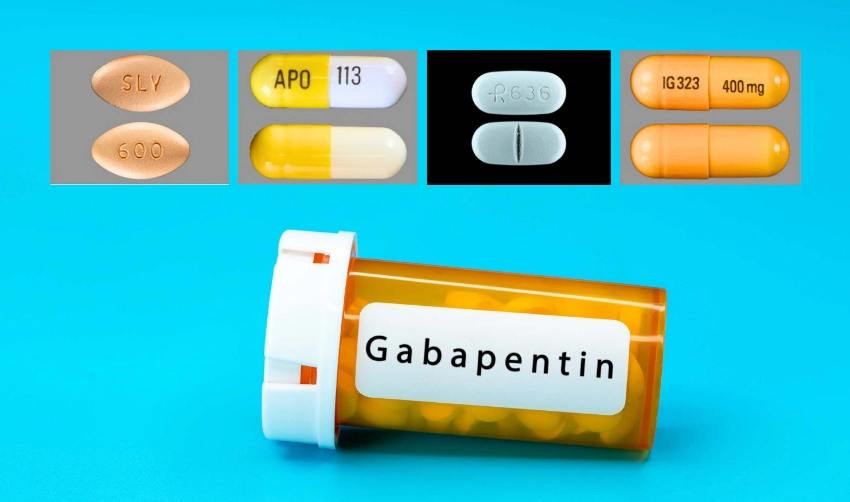
Gabapentin Tablets package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. 2.1 Dosage for Postherpetic Neuralgia. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. 3 days. The recommended 2.2 Dosage for Epilepsy with Partial Onset Seizures 2.3 Dosage Adjustment in Patients with Renal Impairment 2.4 Dosage in Elderly 2.5 Administration Information 3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels. We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels. 5.3 Withdrawal of Gabapentin 5.4 . Tumorigenic Potential 5.5 . Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity 5.6. Laboratory Tests 6 ADVERSE REACTIONS . 6.1 Clinical Trials Experience . 6.2 Postmarketing and Other Experience with other Formulations of Gabapentin . 7 DRUG INTERACTIONS . 7.1 Phenytoin Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly 2.3 Dosage Adjustment in Patients with Renal Impairment Dosage adjustment in patients 12 years of age and older with renal impairment or undergoing hemodialysis is recommended, as follows (see dosing recommendations above for effective doses in each indication): TABLE 1. Gabapentin Dosage Based on Renal Function Renal Function Creatinine Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Administer gabapentin capsules orally with or without food. Gapapentin capsules should be swallowed whole with water. If the gabapentin capsules dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). IN TABLETS. GABAPENTIN tablets, for oral use Initial U.S. Ap. ided doses. The recommended dose is reached by upward titration over a period of approxim. three t. Administer gabapentin orally with or without food. Gabapentin capsules should be swallowed whole with water. If the gabapentin dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). Administer gabapentin three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Pediatric Patients Age 3 to 11 years. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Brand Name: Neurontin. Available in 100 mg, 300 mg, and 400 mg capsules; 600 mg and 800 mg tablets; and oral solution (some products not appropriate for dogs) Background. Gabapentin was originally approved to treat epilepsy in humans. However, gabapentin became more useful as a drug to control nerve pain. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. 3 days. The recommended Read the Medication Guide before you start taking gabapentin and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. What is the most important information I should know about gabapentin?
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |