Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 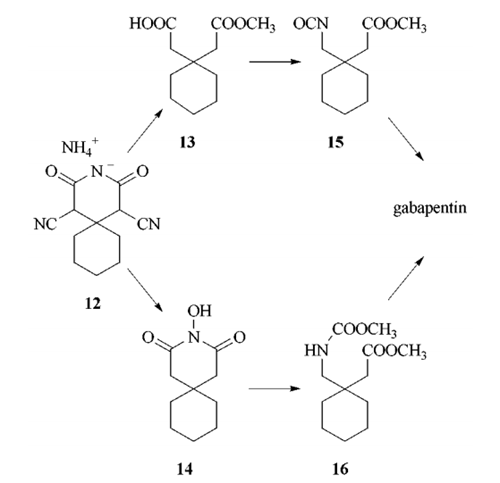 |
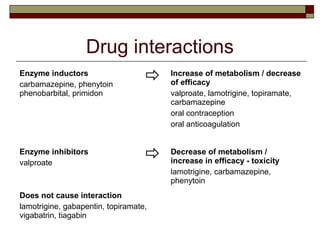
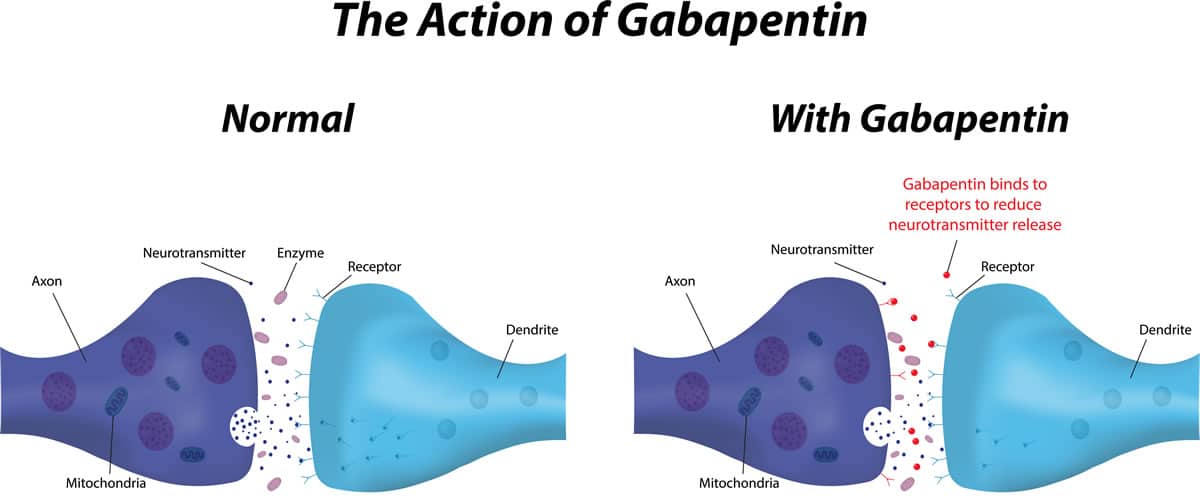
Gabapentin is not protein-bound. A high volume of distribution indicates greater concentration in tissue than in plasma. It is not metabolized and does not induce hepatic enzymes or inhibit metabolism of other antiepileptic drugs. Metabolism. Gabapentin is not appreciably metabolized. Gabapentin enacarbil, the prodrug of gabapentin, is rapidly and efficiently converted to gabapentin by first-pass hydrolysis following oral administration. Elimination Route. Excreted renally as unchanged drug. Half-life. Approximately 5–7 hours. Special Populations Neither drug is metabolized by nor inhibits hepatic enzymes that are responsible for the metabolism of other drugs. Both drugs are excreted renally, with elimination half-lives of approximately 6 hours. Pregabalin and gabapentin both show dose-response relationships in the treatment of postherpetic neuralgia and partial seizures. gabapentin 1800 mg/day to pregabalin 300 mg/day and gabapentin 900 mg/day to hypersensitivity and suppresses medial prefrontal cortical glucose metabolism . in rats with neuropathic pain. Mol Even though gabapentin is a structural GABA analogue, and despite its name, it does not bind to the GABA receptors, does not convert into GABA Tooltip γ-aminobutyric acid or another GABA receptor agonist in vivo, and does not modulate GABA transport or metabolism within the range of clinical dosing. [85] Lin HC, Huang YH, Chao TH, et al. Gabapentin reverses central hypersensitivity and suppresses medial prefrontal cortical glucose metabolism in rats with neuropathic pain. Mol Pain 2014; 10: 63. Crossref Metabolism and Renal Excretion. Gabapentin is not metabolized in humans and is excreted primarily as unchanged drug in the urine. Renal clearance is linearly related to creatinine clearance in both pediatric and adult populations. Therefore, dosage reductions based on declining renal function are recommended (Table 268-1). Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. The chemical structure of gabapentin (Neurontin) is derived by addition of a cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin prevents seizures in a wide variety of models in animals, including generalized tonic-clonic and partial seizures. Gabapentin has no activity at Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. They are not metabolised by the liver and do not affect the cytochrome P450 system, major cytochrome P450 system isoenzymes; however, drug-induced hepatotox-icity has been described in case reports.16 Elimination is mostly done by the kidney and is proportional to the creatinine clearance. Both can be given without regard to food intake since the amount absorbed is unaffected by food. Apparent volumes of distribution for pregabalin and gabapentin are 0.5 and 0.8 L/kg, respectively. Neither drug is metabolized, inhibits the enzymes responsible for the metabolism of other drugs, nor is bound to plasma proteins. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Metabolism. Gabapentin is not appreciably metabolized in humans 16,17 - in humans, metabolites account for less than 1% of an administered dose, with the remainder being excreted as unchanged parent drug in the urine. 5. Route of elimination Metabolism: In humans, gabapentin undergoes minimal metabolic alteration, largely retaining the original structure. Gabapentin does not induce or inhibit CYP enzymes. Also, none of the CYP enzyme inhibitors alter their pharmacokinetics. Gabapentin is approved for the treatment of postherpetic neuralgia (PHN) and epilepsy. The pharmacokinetic (PK) properties of gabapentin, including absorption, distribution, metabolism, and excretion (ADME), were investigated during the development of Neurontin®, an immediate-release (IR) formulatio As a drug, gabapentin was formerly considered as a structural analogue of the inhibitory neurotransmitter γ-aminobutyric acid (GABA). However, preliminary studies proposed that gabapentin did not bind to either GABA-A or GABA-B receptors 11, nor was it transform metabolically into GABA. 12. Gabapentin (gab" a pen' tin) is a structural analogue of gamma-aminobutyric acid (GABA), but demonstrates little or no interaction with GABA receptors and does not appear to alter GABA uptake, synthesis or metabolism. Pharmacokinetics and Drug Metabolism All pharmacological actions following gabapentin administration are due to the activity of the parent compound; gabapentin is not appreciably metabolized in humans. Oral Bioavailability: Gabapentin bioavailability is not dose proportional; i.e., as dose is increased, bioavailability decreases. Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |