Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
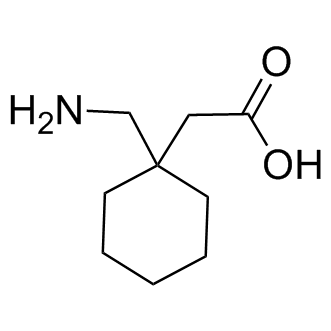
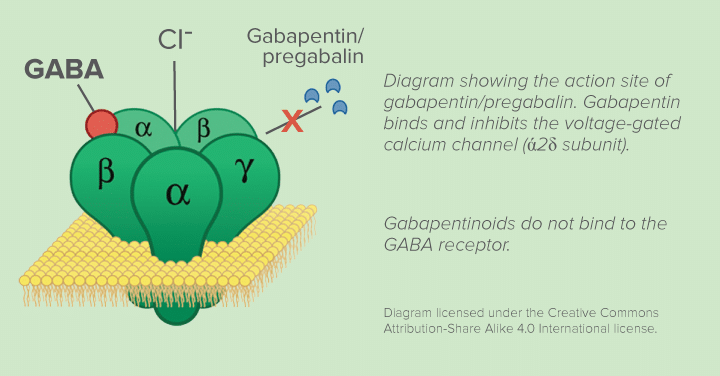
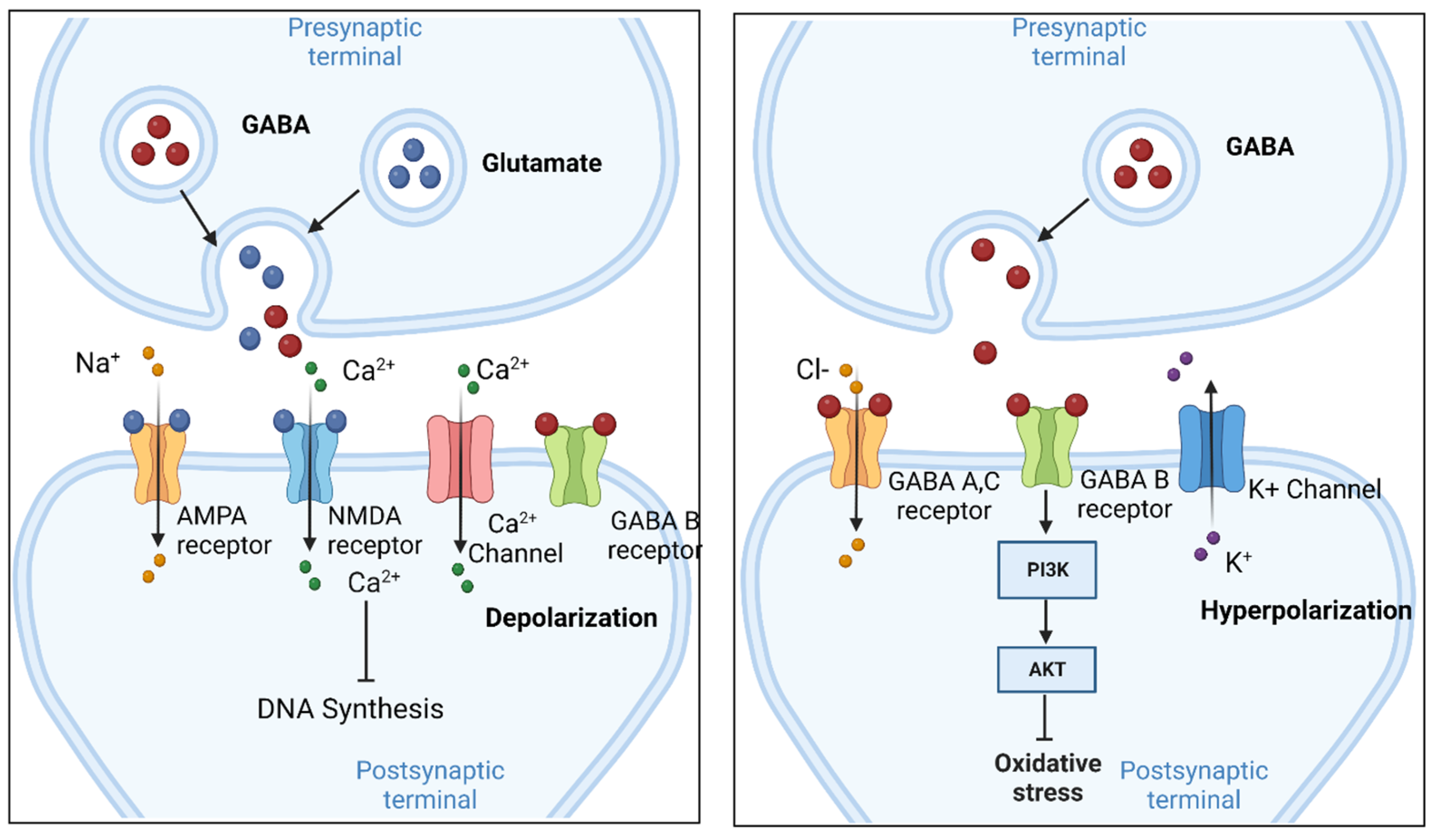
Gabapentin is effective as monotherapy for partial seizures (Beydoun 1999).However, although few studies have compared the effectiveness of gabapentin with other AEDs in the management of partial epilepsy, available evidence suggests that monotherapy with gabapentin is less effective than traditional agents such as carbamazepine and the newer antiepileptic drug lamotrigine (Marson et al. 2007). The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for clinical practice. This article has summarised these key differences. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. Absorption of gabapentin is thought to occur solely via facilitated transport by the LAT1 transporter within the intestines. 5 As this process is saturable, the oral bioavailability of gabapentin is inversely proportional to the administered dose - the oral bioavailability of a 900mg/day regimen is approximately 60%, whereas a 4800mg/day Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10] [7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy , postherpetic neuralgia , and central pain . [ 11 ] As a result, metabolism-related factors do not necessitate dosage alterations of gabapentin and concomitant AEDs after prolonged therapy. The drug is excreted unchanged in urine; plasma clearance is linearly related to creatinine clearance; and dosage is readily adjusted based on renal function. gabapentin 1800 mg/day to pregabalin 300 mg/day and gabapentin 900 mg/day to enhanced recovery pathways, hypersensitivity and suppresses medial prefrontal cortical glucose metabolism . in The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for clinical practice. This article has summarised these key differences. As a drug, gabapentin was formerly considered as a structural analogue of the inhibitory neurotransmitter γ-aminobutyric acid (GABA). However, preliminary studies proposed that gabapentin did not bind to either GABA-A or GABA-B receptors 11, nor was it transform metabolically into GABA. 12 After oral ingestion, gabapentin is rapidly absorbed ( Tmax = 2–3 h) with a bioavailability of <60%. Its volume of distribution is 0.65–1.04 L/kg, and plasma protein binding is 0%. Gabapentin is not metabolized. Approximately 100% of an administered dose is excreted as unchanged gabapentin in urine. Gabapentin is available in two extended-release for-mulations in addition to the immediate release: a gas-tric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neural-gia. Gabapentin is absorbed slowly after oral administration, with maximum plasma concentrations attained within 3-4 hours. Orally administered gabapentin exhibits saturable absorption--a nonlinear (zero-order) process--making its pharmacokinetics less predictable. Plasma concentrations of gabapentin do not increase proportionally with increasing dose. Gabapentin was tested for its effects on seven enzymes in the metabolic pathways of these two neurotransmitters: alanine aminotransferase (AL-T), aspartate aminotransferase (AS-T), GABA aminotransferase (GABA-T), branched-chain amino acid aminotransferase (BCAA-T), glutamine synthetase (Gln-S), glutaminase (GLNase), and glutamate dehydrogenase The pharmacokinetic (PK) properties of gabapentin, including absorption, distribution, metabolism, and excretion (ADME), were investigated during the development of Neurontin®, an immediate-release (IR) formulation of gabapentin that is orally administered three-times daily. . At steady state, it has a half-life of 6–8 h, and is eliminated unchanged by the renal route with a plasma clearance proportional to the creatinine clearance. It is devoid of significant drug-drug interactions when administered with the established AEDs or with oral contraceptives. Gabapentin used as an add-on AED significantly reduced the frequency of partial seizures and secondarily Goldlust A, Su T-Z, Welty De Taylor CP, Oxender DL (1995) Effects of anticonvulsant drug gabapentin on the enzymes in metabolic pathways of glutamate and GABA. Epilepsy Res 22:1–11. Article PubMed CAS Google Scholar Gabapentin crosses several membrane barriers in the body via a specific amino acid transporter (system L) and competes with leucine, isoleucine, valine and phenylalanine for transport. 2. Gabapentin increases the concentration and probably the rate of synthesis of GABA in brain, which may enhance non-vesicular GABA release during seizures. 3. Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |