Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
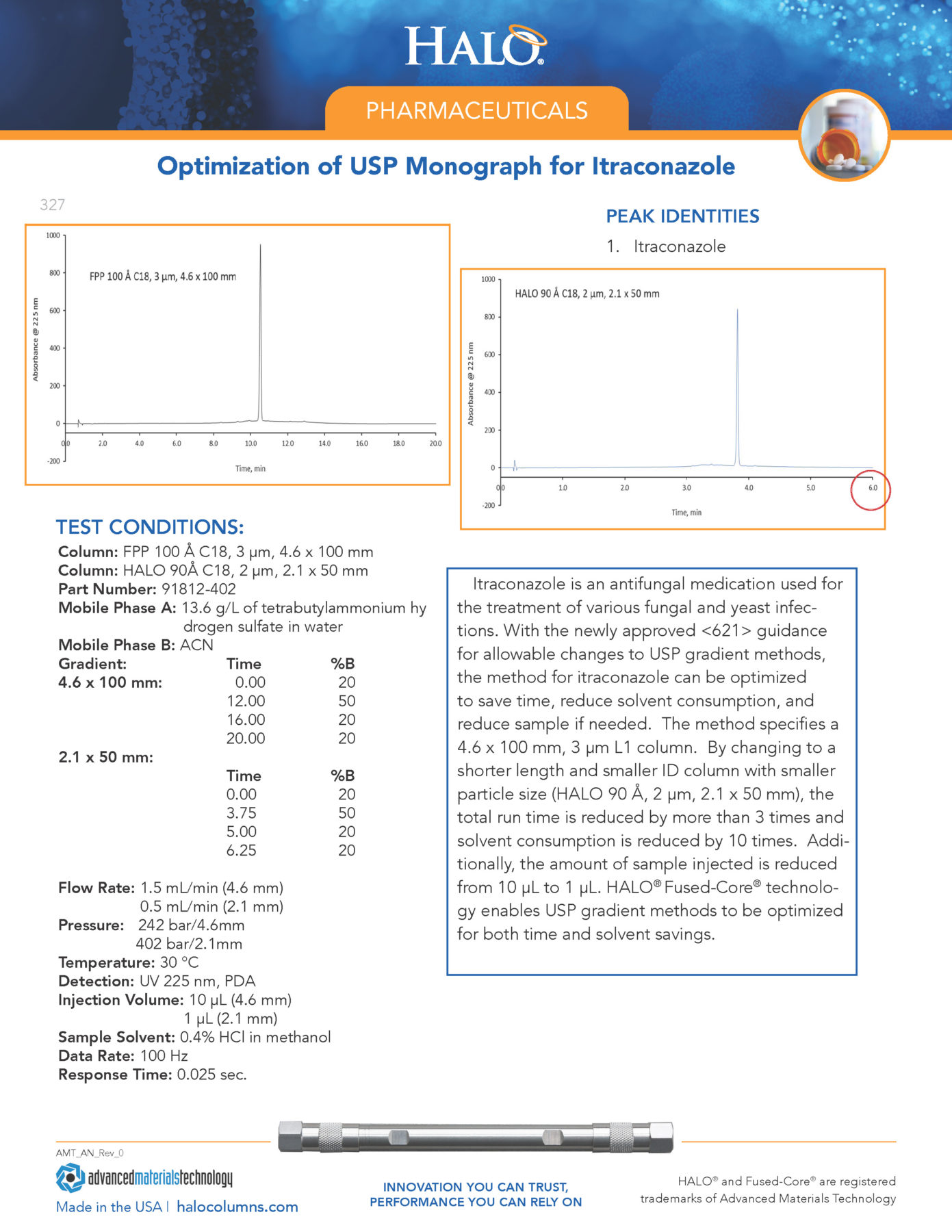
System suitability solution— Dissolve a suitable quantity of USP Gabapentin RS in Diluent, and add an appropriate volume of Impurities solution to obtain a solution containing about 14.0 mg per mL, 0.014 mg per mL, and 0.0084 mg per mL of USP Gabapentin RS, USP Gabapentin Related Compound A RS, and USP Gabapentin Related Compound B RS, respectively. Gabapentin is used in combination with other anticonvulsants for management of partial seizures with or without secondary generalization in adults and children ≥3 years of age. PRODUCT MONOGRAPH PrAPO-GABAPENTIN Gabapentin Capsules Apotex Standard 100 mg, 300 mg and 400 mg Gabapentin Tablets USP 600 mg and 800 mg Antiepileptic Agent APOTEX INC. DATE OF REVISION: 150 Signet Drive April 5, 2018 Toronto, Ontario M9L 1T9 Submission Control No: 214592 United States Pharmacopeia (2024). USP Monographs, Gabapentin Compounded Oral Suspension.USP-NF. Rockville, MD: United States Pharmacopeia. Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. United States Pharmacopeia (2024). USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. USP Working standard solutions, Test solution, Chromatographic Gabapentin Related Compound A RS. system, and Procedure— Proceed as directed for Test 1 . Time: 30 minutes. GABAPENTIN - gabapentin tablet, film coated Sun Pharmaceutical Industries Limited -----Gabapentin Tablets, USP DESCRIPTION Gabapentin tablets, USP are supplied as elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are glyceryl behenate, hydroxypropyl cellulose, low substituted Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly PRODUCT MONOGRAPH Pr MYLAN-GABAPENTIN (Gabapentin Capsules) 100 mg, 300 mg, and 400 mg (Gabapentin Tablets, USP) 600 mg and 800 mg ANTIEPILEPTIC AGENT Mylan Pharmaceuticals ULC 85 Advance Road, Etobicoke, ON M8Z 2S6 Submission Control No.: 202563 Date of Revision: February 14, 2017 Buy [Gabapentin (250 mg)] - CAS [60142-96-3] from USP. * Certain Material Origins (i.e. Animal, Plant, Fish) may require special country importation requirements.USP recommends you contact your country competent authorities to determine if any certifications, permits or licenses may be required prior to ordering.Material Origins are found within the Product under Origin Information. Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. 1.1 Pediatrics Pediatrics (< 18 years of age): Based on the data submitted and reviewed by Health Canada, the safety Standard preparation— Dissolve an accurately weighed quantity of USP Gabapentin RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 4.0 mg per mL. Gabapentin United States Pharmacopeia (USP) Reference Standard; CAS Number: 60142-96-3; Synonyms: 1-(Aminomethyl)-cyclohexaneacetic acid,Neurontin; find USP-1287303 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (2024). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia. Gabapentin Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (2024). USP Monographs, Gabapentin Tablets. USP-NF. Rockville, MD: United States Pharmacopeia. Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. USP 35 Official Monographs / Gabapentin3297 Procedure—Separately inject equal volumes (about 20 µL) of. the Standard solution and the Test solution into the chromato-Gabapentin Capsules graph, record the chromatograms, and measure the responses for the major peaks. [NOTE—Disregard all the peaks having rela-» Gabapentin Capsules contain The Gabapentin Tablets Revision Bulletin supersedes the monograph in USP 32–NF 27 until it is printed in the USP 33–NF 28 First Supplement which will be released 1 February 2010 and becomes official 1 August 2010. Gabapentin Related Compound A RS and USP Gabapentin Re-a 4.6-mm × 25-cm column that contains packing L1. The flow lated Compound B RS in methanol to obtain a solution contain-rate is about 1 mL per minute. TEVA-GABAPENTIN (gabapentin) Product Monograph Page 7 of 32 administration was revealed in reproduction studies in mice at doses up to 62 times, and in rats and rabbits at doses up to 31 times the human dose of 2400 mg/day.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |