Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |
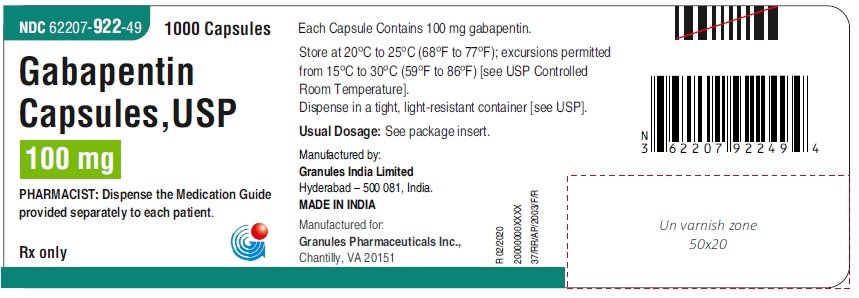
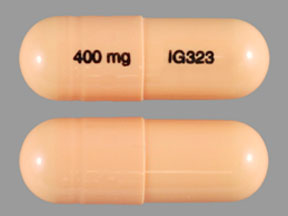
National Drug Code 69097-943 is assigned to gabapentin with active ingredient(s) gabapentin and is labeled by Cipla USA Inc. Controlled Substances - by Controlled Substance Code Number - SUBSTANCE CSCN CSA SCH NARC OTHER NAMES Codeine preparations - 200 mg/(100 ml or 100 gm) V Y Cosanyl,Robitussin A-C,Cheracol,Cerose,Pediacof GABAPENTIN National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or processed by it for commercial distribution. (See Section 510 of the Federal Food, Drug, and Cosmetic The NDC code 45963-556 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Actavis Pharma, Inc.. The product's dosage form is capsule and is administered via oral form. National Drug Codes 68001-0006 Gabapentin National Drug Codes Code Information . 68001-0006 - NDC® Code. View information about the NDC, including active The NDC Packaged Code 67877-222-01 is assigned to a package of 100 capsule in 1 bottle of Gabapentin, a human prescription drug labeled by Ascend Laboratories, Llc. The product's dosage form is capsule and is administered via oral form. Manufacturers of Gabapentin that have been granted an NDC (National Drug Code). Gabapentin is a Oral Tablet in the Human Prescription Drug category. It is labeled and distributed by Teva Pharmaceuticals Usa, Inc.. The primary component is Gabapentin. Sample Package? 0093-4444 National Drug Code registration, ingredients, and packaging details. List of products in the National Drug Code with proprietary name gabapentin. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. National Drug Codes Number: 38779-2461-1. Drug Non Proprietary Name: Gabapentin. Drug Trade Name: . The NDC code 69097-943 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Cipla Usa Inc.. The product's dosage form is capsule and is administered via oral form. National Drug Codes Number: 71093-161-04. Drug Non Proprietary Name: Gabapentin. Drug Trade Name: Gabapentin. Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. National Drug Codes Number: 68094-489-60. Drug Non Proprietary Name: Gabapentin. Drug Trade Name: Gabapentin. GABAPENTIN National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or processed by it for commercial distribution. (See Section 510 of the Federal Food, Drug, and Cosmetic National Drug Codes Number: 52427-890-60. Drug Non Proprietary Name: Gabapentin. Drug Trade Name: Gralise. GABAPENTIN National Drug Code Directory The Drug Listing Act of 1972 requires registered drug establishments to provide the Food and Drug Administration (FDA) with a current list of all drugs manufactured, prepared, propagated, compounded, or processed by it for commercial distribution. (See Section 510 of the Federal Food, Drug, and Cosmetic National Drug Codes Number: 65162-698-90. Drug Non Proprietary Name: Gabapentin. Drug Trade Name: Gabapentin. The NDC Code 80425-0158-1 is assigned to “Gabapentin ” (also known as: “Gabapentin”), a human prescription drug labeled by “Advanced Rx Pharmacy of Tennessee, LLC”. The product's dosage form is tablet, film coated, and is administered via oral form. 60429-739-05 Gabapentin Labeler Name Golden State Medical Supply, Inc. Name of Company corresponding to the labeler code segment of the ProductNDC. NDC Package Code 60429-739-05 The labeler code, product code, and package code segments of the National Drug Code number, separated by hyphens. Asterisks are no longer used or included
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |