Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
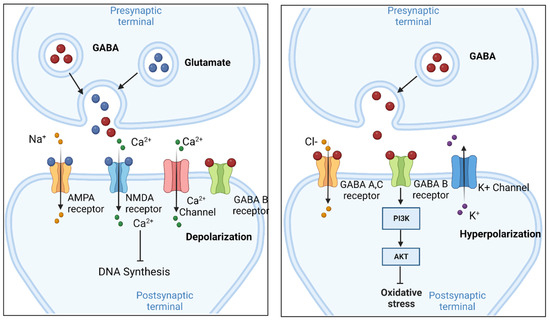
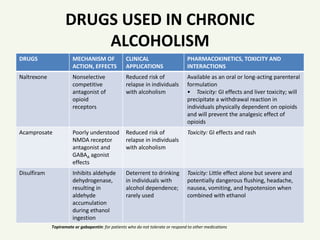
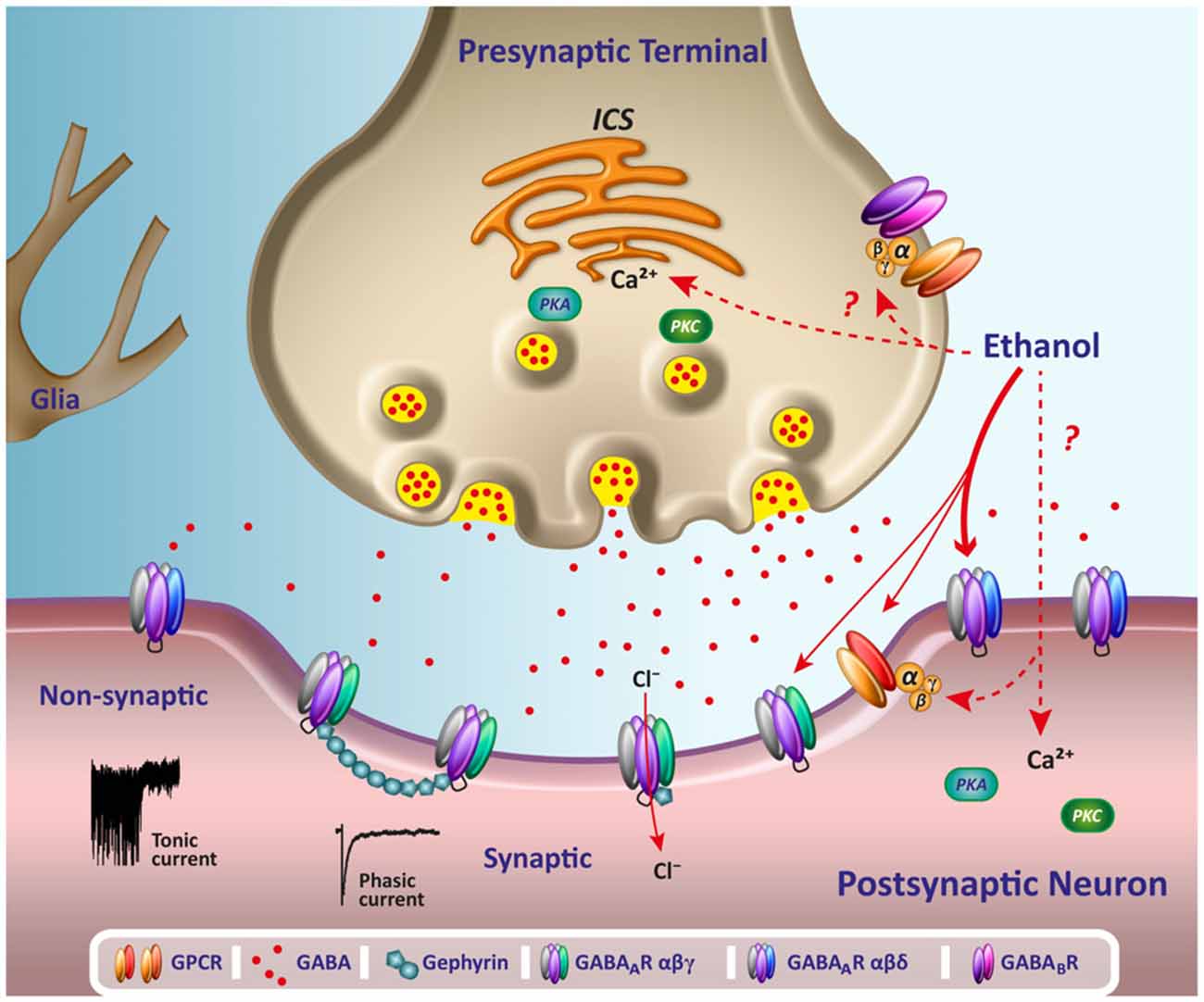
Chen et al. show that α2δ-1, through its C terminus, physically interacts with NMDA receptors and promotes synaptic expression of α2δ-1-NMDA receptor complexes in neuropathic pain. Gabapentin reduces neuropathic pain primarily by targeting α2δ-1-bound NMDA receptors. However, receptor binding studies fail to detect any specific interactions between gabapentin and the NMDA receptor complex , including the strychnine-insensitive glycine site . In the absence of specific binding data, it is unlikely that gabapentin has its effect by means of interacting with the NMDA receptor. Several mechanisms of gabapentin have been proposed after neuropathy including an inhibition of NMDA receptors, inhibition of sodium currents and reducing β4a subunit mediated VGCC trafficking (Hara and Sata 2007; Mich and Horne 2008; Yang et al. 2009). Background and aims The clinical management of neuropathic pain remains a challenge. We examined the interaction between gabapentin and NMDA receptor antagonists dextromethrophan and MK-801 in alleviating neuropathic pain-like behaviors in rats after spinal cord or sciatic nerve injury. Methods Female and male rats were produced with Ischemic spinal cord injury and sciatic nerve injury Cell Reports Article The a2d-1-NMDA Receptor Complex Is Critically Involved in Neuropathic Pain Development and Gabapentin Therapeutic Actions Jinjun Chen,1,2,5 Lingyong Li,1,5 Shao-Rui Chen,1,5 Hong Chen,1 Jing-Dun Xie,1,3 Rita E. Sirrieh,4 David M. MacLean,4 Gabapentin has also been shown to induce modulate other targets including transient receptor potential channels, NMDA receptors, protein kinase C and inflammatory cytokines. It may also act on supra-spinal region to stimulate noradrenaline mediated descending inhibition, which contributes to its anti-hypersensitivity action in neuropathic pain. Gabapentinoids inhibit the joint action of voltage-gated calcium channel (VGCC) α2δ subunits in conjunction with the n -methyl- d -aspartate (NMDA) receptor, with subsequent downregulation of VGCC expression and excitatory neurotransmitter release, and possibly synaptogenesis as well, through actions on thrombospondins. However, receptor binding studies have failed to demonstrate a direct binding site for gabapentin at the NMDA receptor . The α 2 δ subunit of the voltage-dependent calcium channel is a binding site for gabapentin and the S -isomer of pregabolin ( S -(+)-3-isobutylgaba) [ 4, 33, 37, 41 ]. Gabapentin or an α2δ-1 C terminus-interfering peptide normalizes NMDAR synaptic targeting and activity increased by nerve injury. Thus, α2δ-1 is an NMDAR-interacting protein that increases NMDAR synaptic delivery in neuropathic pain. Gabapentin or an α2δ-1 C terminus-interfering peptide normalizes NMDAR synaptic targeting and activity increased by nerve injury. Thus, α2δ-1 is an NMDAR-interacting protein that increases NMDAR synaptic delivery in neuropathic pain. Gabapentinoids reduce neuropathic pain by inhibiting forward trafficking of α2δ-1-NMDAR complexes. Background and aims The clinical management of neuropathic pain remains a challenge. We examined the interaction between gabapentin and NMDA receptor antagonists dextromethrophan and MK-801 in alleviating neuropathic pain-like behaviors in rats after spinal cord or sciatic nerve injury. Conversely, knockdown or removal of Cacna2d1 normalized the increased NMDA receptor activity induced by nerve injury. Further investigation revealed that α2-δ1 forms a complex with NMDA receptors in both rodent and human spinal cords, predominantly through its C terminus, promoting the surface trafficking and synaptic targeting of NMDA receptors. α2δ-1 (encoded by the Cacna2d1 gene) is a newly discovered NMDA receptor-interacting protein and is the therapeutic target of gabapentinoids (e.g., gabapentin and pregabalin) frequently used for treating patients with neuropathic pain. Patch-clamp studies revealed that gabapentin significantly inhibited the NMDA receptor–activated ion current in dissociated hippocampal CA1 neurons, resulting in suppression of glutamate-induced neuronal injury. Chen et al. show that α2δ-1, through its C terminus, physically interacts with NMDA receptors and promotes synaptic expression of α2δ-1-NMDA receptor complexes in neuropathic pain. Gabapentin reduces neuropathic pain primarily by targeting α2δ-1-bound NMDA receptors. A and B, Representative gel images (A) and quantification (B) show the effects of FK506 treatment with and without 100 μM gabapentin on protein levels of α2δ-1, GluN1(glutamate NMDA receptor 1), and α2δ-1–GluN1 complexes in PVN synaptosomes (n=6 samples per group; each sample included PVN tissues from 2 male rats). Chen et al. recently discovered a novel mechanism through which gabapentinoids relieve neuropathic pain 7. They found that α2δ1 binds to N-methyl-D-aspartate (NMDA) receptor in both rodent and human to enhance its activity in spinal cord after nerve injury, and gabapentin prevents α2δ1-mediated NMDA receptor (NMDAR) hyperactivity 7. Evidence linking gabapentin to the NMDA receptor follows research demonstrating the reversal of the antihyperalgesic effect of gabapentin by d-serine, an agonist at the NMDA-glycine binding site [33, 34, 36, 37]. Gabapentin has also been shown to induce modulate other targets including transient receptor potential channels, NMDA receptors, protein kinase C and inflammatory cytokines. It may also act on supra-spinal region to stimulate noradrenaline mediated descending inhibition, which contributes to its anti-hypersensitivity action in neuropathic pain. Gabapentin reduces neuropathic pain primarily by targeting a2d-1-bound NMDA receptors. a2d-1, commonly known as a voltage-activated Ca2+ channel subunit, is a binding site of gabapentinoids used to treat neuropathic pain and epilepsy. How-ever, it is unclear how a2d-1 contributes to neuro-pathic pain and gabapentinoid actions.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |