Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
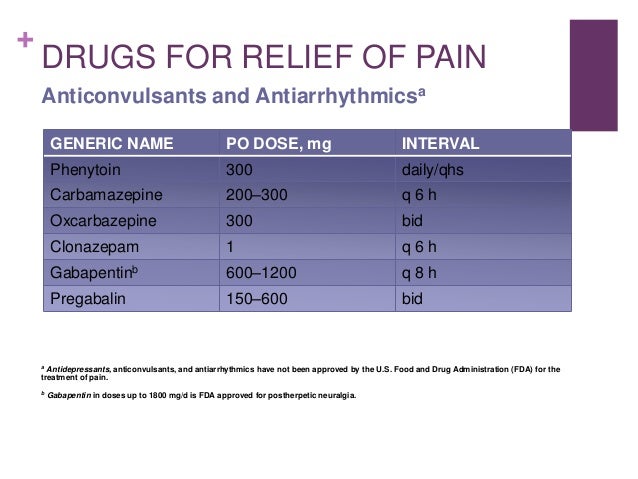
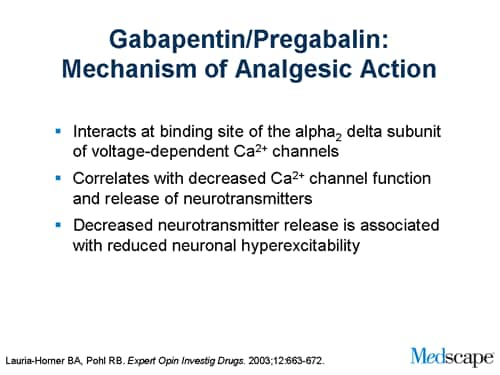
FDA Approved Labeling Text dated 03/01/2011 Page 3 . Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Finally, gabapentin is likely used for RLS or PLMD in lieu of the costly, brand-name-only gabapentin enacarbil (Horizant), 39 despite package recommendations stating that these agents are not interchangeable due to varying pharmacokinetic properties. 39 Prior to market availability of gabapentin enacarbil, which does carry an FDA-approved Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic Inform patients that, should they divide the scored 600 mg or 800 mg NEURONTIN tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividing the scored tablet should be discarded. Reference ID: 4168942 This label may not be the latest approved by FDA. Gabapentin and pregabalin are FDA-approved for a variety of conditions, including seizures, nerve pain, and restless legs syndrome. Our evaluation shows that the use of these medicines, often “Off-label" uses of gabapentin are uses that have not been approved by the FDA and are not found in the package insert, but may have been accepted for use by healthcare providers based on clinical use. FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in patients who use gabapentanoids with opioid pain medicines or other drugs that depress the Gabapentin (Neurontin) is FDA-approved to treat specific types of nerve pain and seizures. It’s also sometimes used to treat other health conditions. These include restless leg syndrome, anxiety, and alcohol withdrawal. Gabapentin isn’t a controlled substance according to the federal government. Gabapentin . 7 DRUG INTERACTIONS . 7.1 Phenytoin . 7.2 Carbamazepine . 7.3 Valproic Acid . 7.4 Phenobarbital . This label may not be the latest approved by FDA. The authors concluded that gabapentin is associated with reduction in acute pain associated with postherpetic neuralgia and peripheral diabetic neuropathy (the later indication is not approved by the FDA), and that there is limited evidence to support the use of gabapentin for other types of neuropathic pain and pain disorders. 1 This Editorial The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gabapentin (brand names: Aclonium, Equipax, Gantin, Gabarone, Gralise, Neurontin, Neurostil, Progresse) is a medicine used in dogs and cats. it’s not FDA-approved for use in animals To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. FDA approved labeling text (dated 10/12/00) Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. One such drug, gabapentin (Neurontin), received approval by the U.S. Food and Drug Administration (FDA) in 1993 as an adjunct medicine for partial seizures and additional FDA approval in 2002 for Gabapentin is an anticonvulsant (antiseizure) medication approved by the FDA to treat several conditions. Doctors sometimes prescribe gabapentin "off-label" to treat other conditions as well. A 2022 report stated that gabapentin was among the 10 most commonly prescribed medications in the U.S. Why isn’t gabapentin FDA approved for common uses? Although gabapentin has been shown to be effective in clinical trials for conditions like neuropathic pain, the manufacturer Pfizer has never formally applied for additional FDA approvals. 17.5 Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity . 17.6 Lack of Interchangeability With Gabapentin 17.7 Dosing Instructions . 17.8 Alcohol * Sections or subsections omitted from the full prescribing information are not listed. 1. Reference ID: 4584082 . This label may not be the latest approved by FDA.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |