Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
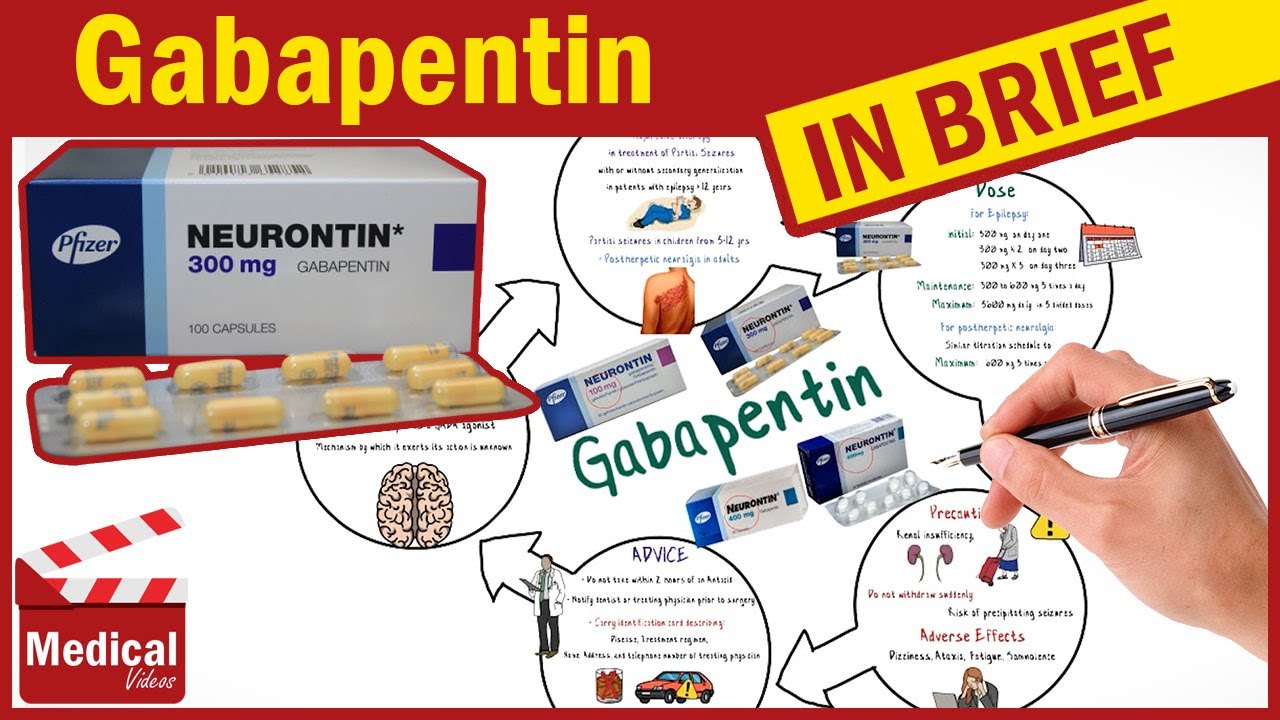
For gabapentin, the only pain-related indication approved by the US Food and Drug Administration (FDA) is postherpetic neuralgia. For pregabalin, FDA-approved indications related to pain are limited to postherpetic neuralgia, neuropathic pain associated with diabetic neuropathy or spinal cord injury, and fibromyalgia. Gabapentin’s off-label uses—meaning prescribing gabapentin for a problem not on the FDA approval list for this medication—that physicians are finding include: Alcohol withdrawal; Anxiety; Cocaine withdrawal; Fibromyalgia; Headaches; Hiccups; Hot flashes; Hyperhidrosis; Insomnia; Migraines; Mood disorders; Pain syndromes; Peripheral Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. Methods: National Ambulatory Care Medical Survey data (2011-2016) were used to identify encounters involving gabapentin (gabapentin visits) for adults (ages ≥18) (N=5,732). FDA-approved uses and off-label psychiatric use indications were identified with ICD-9-CM and ICD-10-CM diagnosis codes. CNS-D drugs examined were opioids, benzodiazepines Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. SUMMARY: Gabapentin is approved by the U.S. Food and Drug Administration (FDA) for adjunctive therapy in treatment of partial seizures and postherpetic neuralgia. Various off-label (unapproved) uses have been reported, and the use of gabapentin for off-label purposes has reportedly exceeded use for FDA-approved indications. Like gabapentin, it is sometimes used with opiates, with toxic or even lethal results. Similarly, when in combination with alcohol or nervous system depressants, there is the possibility of greater toxicity. Choosing gabapentin and pregabalin: These drugs are widely used off-label as an alternative to benzodiazepines for anxiety disorders. Perhaps one of the more promising off-label uses for Gabapentin is for the treatment of anxiety disorders. There is mounting evidence that Gabapentin may be an effective intervention for various types of anxiety including: generalized anxiety disorder, social anxiety disorder, and panic disorder. This study examined off-label use of gabapentin for psychiatric indications and its concomitant use with CNS-D prescription drugs in a nationally representative sample of ambulatory care office visits. Less than 1% of outpatient gabapentin use was for FDA-approved indications. There is minimal or no evidence for the use of gabapentin as an off-label therapy for other types of neuropathic pain, low-back pain, radiculopathy, or fibromyalgia. In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 We examined clinical trials of gabapentin (Neurontin, Pfizer) for off-label use for migraine prophylaxis, bipolar disorders, neuropathic pain, or nociceptive pain. Outcomes described in published Off-label prescribing does not necessarily signify that the medication is being used improperly, and in some cases, reliable research might validate its use. Nonetheless, prescribing gabapentin for off-label use might also result in negative consequences, including adverse drug effects, liability concerns, and a lack of reimbursement due to the “Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids Vedula SS, Bero L, Scherer RW, et al. Outcome reporting in industry-sponsored trials of gabapentin for off-label use. N Engl J Med. 2009;361(20):1963-1971. Goodman CW, Brett AS. A clinical Off-label gabapentin (Neurontin) got a bad rep when it missed the mark in bipolar disorder, but there may be something worth salvaging in this drug. Here, we weigh its pros and cons for anxiety, substance use disorders, sleep, pain, and hot flashes, and compare it to its underutilized cousin, pregabalin (Lyrica). We selected gabapentin (Neurontin), a medication reported to be widely used off label, as a specific example to explore specialist physicians' experiences with off-label prescribing. Observations: This report summarizes the limited published evidence to support off-label gabapentinoid uses, describes clinical cases in which off-label use is problematic, and notes how review articles and guidelines tend to overstate gabapentinoid effectiveness. In addition to being used to treat pain, gabapentin is used off label to treat anxiety, alcohol use disorder (AUD), alcohol withdrawal, depression, substance use disorders (SUDs), sleep problems, and more.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |