Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
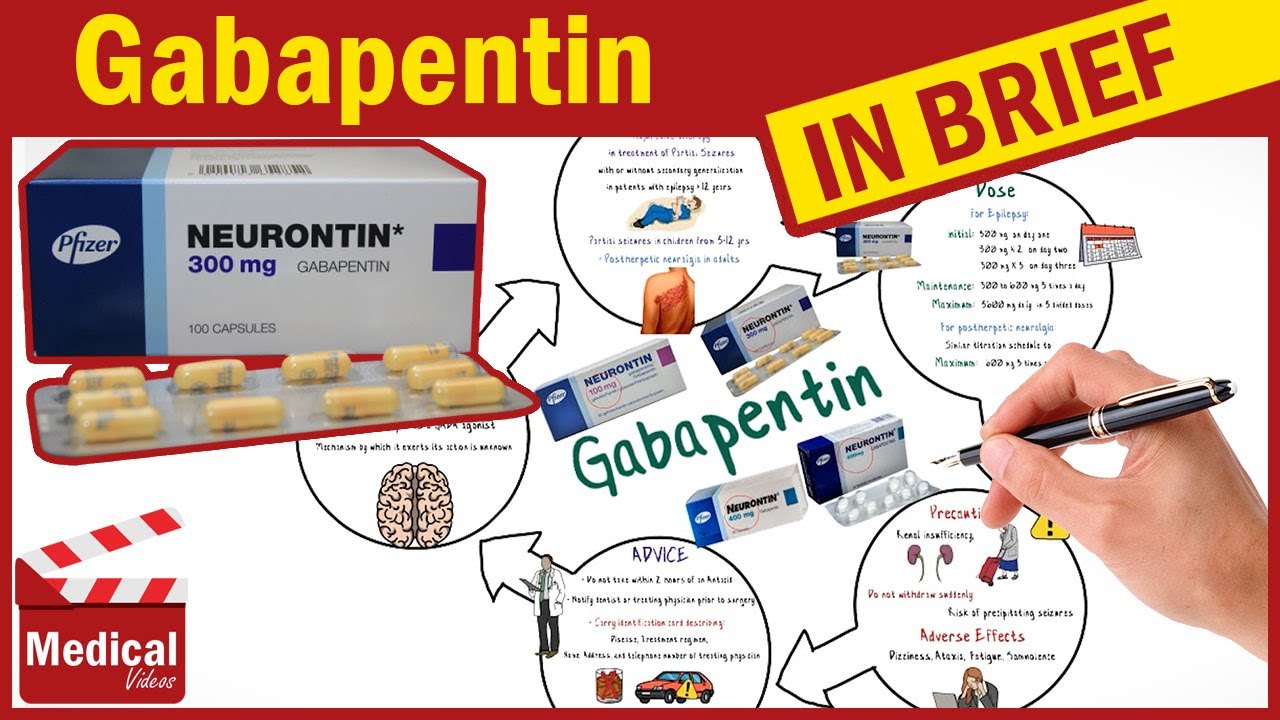
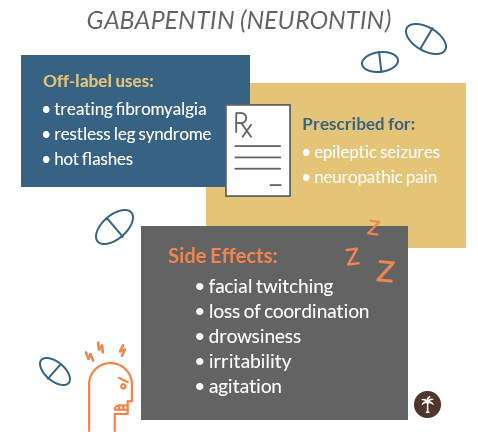
In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 Here is a list of some of the common off-label uses: Anxiety Disorders : Gabapentin is sometimes used to manage generalized anxiety disorder (GAD) and social anxiety, especially in patients who don’t respond well to traditional anti-anxiety medications. “To prescribe gabapentinoids responsibly, clinicians should be familiar with the published evidence that addresses off-label use of these drugs,” the authors wrote. 1 To that end, they Gabapentin's off-label uses are diverse, ranging from pain management to psychiatric and substance use disorders. While it shows promise in certain areas, such as anxiety disorders and alcohol use disorder, the evidence is less convincing for other conditions like depression and PTSD. While Gabapentin is FDA-approved for partial seizures and postherpetic neuralgia, its off-label uses are more extensive, especially in psychiatry. Gabapentin for anxiety disorders is notable, with doses between 900 and 3,600 milligrams per day showing effectiveness in reducing symptoms. Off-label: It is estimated that approximately 9/10 prescriptions for Gabapentin are “off-label” or for conditions that the drug isn’t approved to treat. Off-label prescriptions have a reduce chance of actually working for the treatment of anxiety. The rise in gabapentin prescribing is multifactorial but thought to be due in part to efforts by the pharmaceutical industry to promote the use of the medication for off-label uses. (In 2004, the manufacturer of Neurontin, Pfizer, pleaded guilty to multiple counts of illegally promoting the off-label use of gabapentin, resulting in nearly $430 off-label experimentation with gabapentin commenced for neuropathic pain, bipolar disorder, and migraine prophylaxis (6). The sustained upward trend in off-label prescribing of gabapentin is concerning, considering the minimal high-quality evidence for many off-label uses. Recent attention to off-label use of gabapentin is largely Use of gabapentin has increased since 2008; use of pregabalin has remained steady. Between 2017 and 2021, most patients using gabapentinoids reported having musculoskeletal pain and diabetes. We selected gabapentin (Neurontin), a medication reported to be widely used off label, as a specific example to explore specialist physicians' experiences with off-label prescribing. Off-label gabapentin (Neurontin) got a bad rep when it missed the mark in bipolar disorder, but there may be something worth salvaging in this drug. Here, we weigh its pros and cons for anxiety, substance use disorders, sleep, pain, and hot flashes, and compare it to its underutilized cousin, pregabalin (Lyrica). This Special Communication summarizes the limited published evidence to support off-label gabapentinoid uses, describes clinical cases in which off-label use is problematic, and notes how review articles and guidelines tend to overstate gabapentinoid effectiveness. The rise in gabapentin prescribing is multifactorial but thought to be due in part to efforts by the pharmaceutical industry to promote the use of the medication for off-label uses. (In 2004, the manufacturer of Neurontin, Pfizer, pleaded guilty to multiple counts of illegally promoting the off-label use of gabapentin, resulting in nearly $430 Off-label uses for gabapentin. Doctors often prescribe gabapentin off-label to treat conditions such as: pain from diabetic neuropathy, which is numbness or uncomfortable tingling caused by nerve damage from diabetes; nerve pain in the neck and back from conditions such as sciatica, a painful compression of the sciatic nerve for an off-label use. Examples of Off-Label Medication Use for Mental Health Conditions . Amitriptyline • Insomnia • Posttraumatic stress disorder (PTSD) Clonidine • Smoking cessation • Excessive saliva caused by clozapine Gabapentin • Alcohol dependence • Social anxiety Prazosin • Post traumatic stress disorder For the evidence addressing off-label gabapentinoid use, their noteworthy findings included: 1. The evidence is mixed at best for the use of gabapentin for painful diabetic neuropathy. 2. There are few studies of gabapentinoids for nondiabetic neuropathies. 3. The evidence does not support gabapentinoid therapy for low back pain or radiculopathy. Methods: National Ambulatory Care Medical Survey data (2011-2016) were used to identify encounters involving gabapentin (gabapentin visits) for adults (ages ≥18) (N=5,732). FDA-approved uses and off-label psychiatric use indications were identified with ICD-9-CM and ICD-10-CM diagnosis codes. CNS-D drugs examined were opioids, benzodiazepines Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. Gabapentin’s off-label uses—meaning prescribing gabapentin for a problem not on the FDA approval list for this medication—that physicians are finding include: Alcohol withdrawal; Anxiety; Cocaine withdrawal; Fibromyalgia; Headaches; Hiccups; Hot flashes; Hyperhidrosis; Insomnia; Migraines; Mood disorders; Pain syndromes; Peripheral Known off-label uses where doctors prescribe gabapentin include as a treatment for hot flashes, restless leg syndrome, mood disorders, anxiety, and to diminish nerve pain related to diabetes or
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |