Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
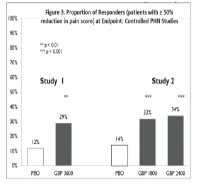
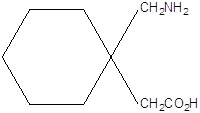
Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. Gabapentin is known as an anticonvulsant or antiepileptic drug. Gabapentin Oral Solution is supplied as follows: 250 mg/5 mL oral solution; Clear, colorless to slightly yellow solution; each 5 mL of oral solution contains 250 mg of gabapentin; available in: Bottles containing 470 mL, 5 mL unit dose, and 6 mL unit dose in trays of ten cups. Storage. Store refrigerated, 2° - 8°C (36° - 46°F) Rx Only Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults ( 1) Gabapentin Oral Solution is a prescription medicine used to treat: z Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. • 600 mg: white elliptical film-coated scored tablets debossed with “NT” and “16” on one side • 800 mg: white elliptical film-coated scored tablets debossed with “NT” and “26” on one side Oral solution • 250 mg per 5 mL (50 mg per mL), clear colorless to slightly yellow solution . 4 CONTRAINDICATIONS Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Gabapentin by is a Prescription medication manufactured, distributed, or labeled by Taro Pharmaceuticals U.S.A., Inc., Taro Pharmaceutical Industries Ltd.. Drug facts, warnings, and ingredients follow. Rx only. Gabapentin Oral Solution is supplied as oral solution containing 250 mg/5 mL of gabapentin. Solution, Oral: Neurontin: 250 mg/5 mL (470 mL); 250 mg/5 mL (470 mL) [strawberry anise flavor] gabapentin 400 mg; gabapentin 300 mg; gabapentin 100 mg Oral solution: 250 mg per 5 mL (50 mg per mL), clear colorless to slightly yellow solution 4 CONTRAINDICATIONS Gabapentin Oral Solution is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms Gabapentin Oral Solution is supplied as follows: 250 mg per 5 mL oral solution: Clear colorless to slightly yellow solution; each 5 mL of oral solution contains 250 mg of gabapentin; available in: 5 mL unit dose cups, boxes of 40: NDC 42192-608-45; 6 mL unit dose cups, boxes of 40: NDC 42192-608-40; Bottles containing 470 mL: NDC 42192-608-16 250 mg per 5 mL oral solution: Clear colorless to slightly yellow solution; each 5 mL of oral solution contains 250 mg of gabapentin; available in: Glass bottles containing 470 mL: NDC 0071-2012-23 Bottles containing 470 mL: NDC 0071-2012-44, NDC 0071-2012-47 Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Gabapentin Oral Solution 250 mg / 5 mL. Read the Medication Guide before you start taking Gabapentin Oral Solution and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults (1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary If the gabapentin dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). 3 DOSAGE FORMS AND STRENGTHS Oral solution: 250 mg per 5 mL (50 mg per mL), clear colorless to slightly yellow solution Gabapentin oral solution, Amneal, 250 mg/5 mL, 473 mL bottle, NDC 65162-0698-90 Gabapentin oral solution, Mylan (Viatris), 250 mg/5 mL, 470 mL bottle, NDC 59762-5050-07 Estimated Resupply Dates Oral solution: 250 mg per 5 mL (50 mg per mL), clear pale yellow to yellow solution. Gabapentin oral solution is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 250 mg per 5 mL oral solution: Clear colorless to slightly yellow solution; each 5 mL of oral solution contains 250 mg of gabapentin; available in: Bottles containing 470 mL: NDC 31722-069-47 Capsules: 100 mg, 300 mg, and 400 mg (3) Tablets: 600 mg, and 800 mg (3) Oral Solution: 250 mg/5mL (3) Drug Reaction with Eosinophilia and Systemic Symptoms (Multiorgan hypersensitivity): Discontinue if alternative etiology is not established (5.1) Anaphylaxis and Angioedema: Discontinue and evaluate patient immediately (5.2)
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |