Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
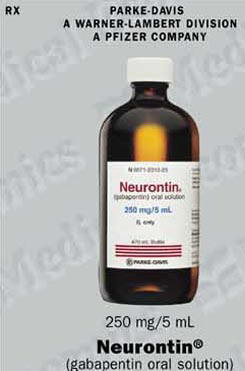 |  |
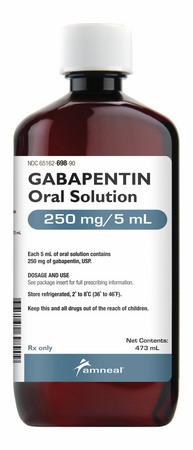
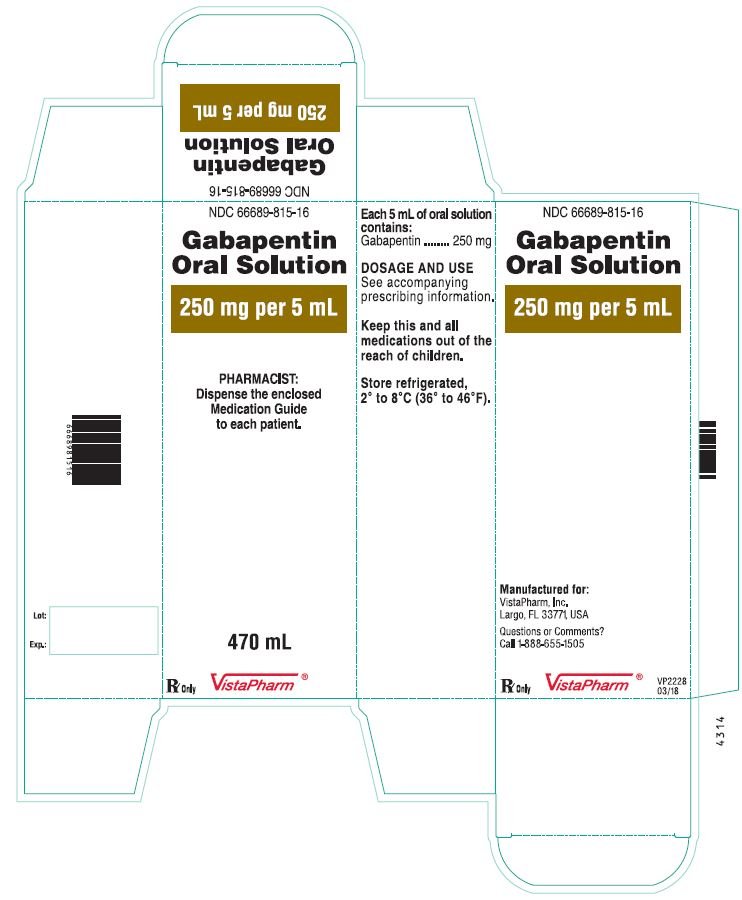
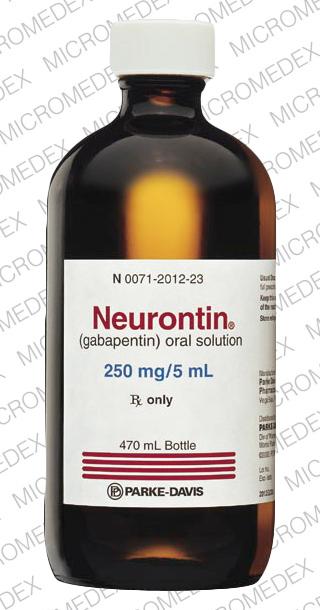
Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Oral solution: 250 mg per 5 mL (50 mg per mL), clear pale yellow to yellow solution. Gabapentin oral solution is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. Gabapentin is available as Gralise, Neurontin, and generic gabapentin in the following dosage forms that are taken by mouth. 100 mg, 300 mg, 400 mg oral capsules; 250 mg/5 mL oral solution Gabapentin Oral Solution is a prescription medicine used to treat: z Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. Oral solution: 250 mg per 5 mL (50 mg per mL), clear colorless to slightly yellow solution 4 CONTRAINDICATIONS Gabapentin Oral Solution is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults (1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Stopping Gabapentin Oral Solution suddenly can cause serious problems. Gabapentin Oral Solution can cause serious side effects including: 1. Like other antiepileptic drugs, Gabapentin Oral Solution may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Gabapentin oral solution is supplied as follows: 250 mg per 5 mL oral solution: Clear colorless to slightly yellow solution; each 5 mL of oral solution contains 250 mg of gabapentin; available in: Bottles containing 470 mL: NDC 31722-069-47. Store gabapentin oral solution refrigerated, 2°C to 8°C (36°F to 46°F). Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. Gabapentin is known as an anticonvulsant or antiepileptic drug. Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of 250 mg per 5 mL oral solution: Clear colorless to slightly yellow solution; each 5 mL of oral solution contains 250 mg of gabapentin; available in: Glass bottles containing 470 mL: NDC 0071-2012-23 Bottles containing 470 mL: NDC 0071-2012-44, NDC 0071-2012-47 SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults ( 1) NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Coadministration of gabapentin oral solution (125 to 500 mg; N=48) decreases hydrocodone (10 mg; N=50) C max and AUC values in a dose-dependent manner relative to administration of hydrocodone alone; C max and AUC values are 3% to 4% lower, respectively, after administration of 125 mg gabapentin oral solution and 21% to 22% lower, respectively effectively. See full prescribing information for GABAPENTIN. Gabapentin capsules, for oral use Gabapentin tablets, for oral use Gabapentin oral solution Initial U.S. Approval: 1993 INDICATIONS AND USAGE Gabapentin is indicated for: • • DOSAGE AND ADMINISTRATION • • • DOSAGE FORMS AND STRENGTHS • • • CONTRAINDICATIONS SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) Neuropathic pain: Limited data available: Oral: Immediate release: Children and Adolescents: Initial: 5 mg/kg/dose up to 300 mg at bedtime; day 2: Increase to 5 mg/kg/dose twice daily (up to 300 mg twice daily); day 3: Increase to 5 mg/kg/dose 3 times daily (up to 300 mg 3 times daily); further titrate with dosage increases (not frequency) to How should I store Gabapentin Oral Solution? •Store Gabapentin Oral Solution in the refrigerator between 36°F to 46°F (2°C to 8°C). Keep gabapentin and all medicines out of the reach of children. General information about the safe and effective use of gabapentin Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long term clinical study. Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |