Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
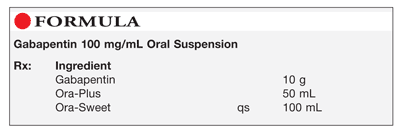
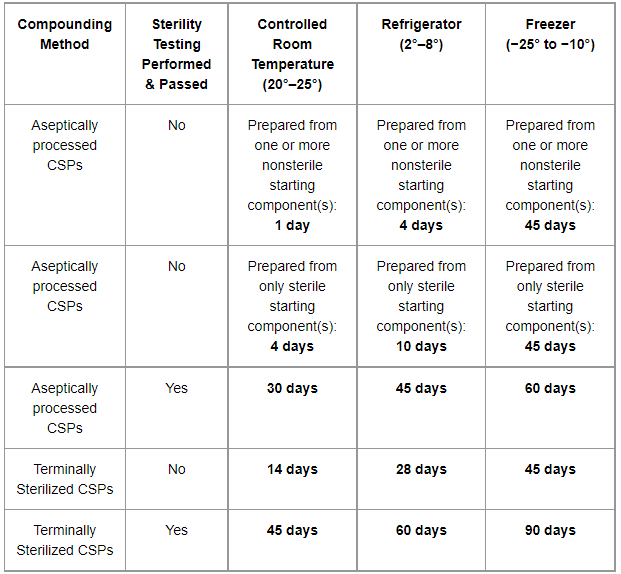
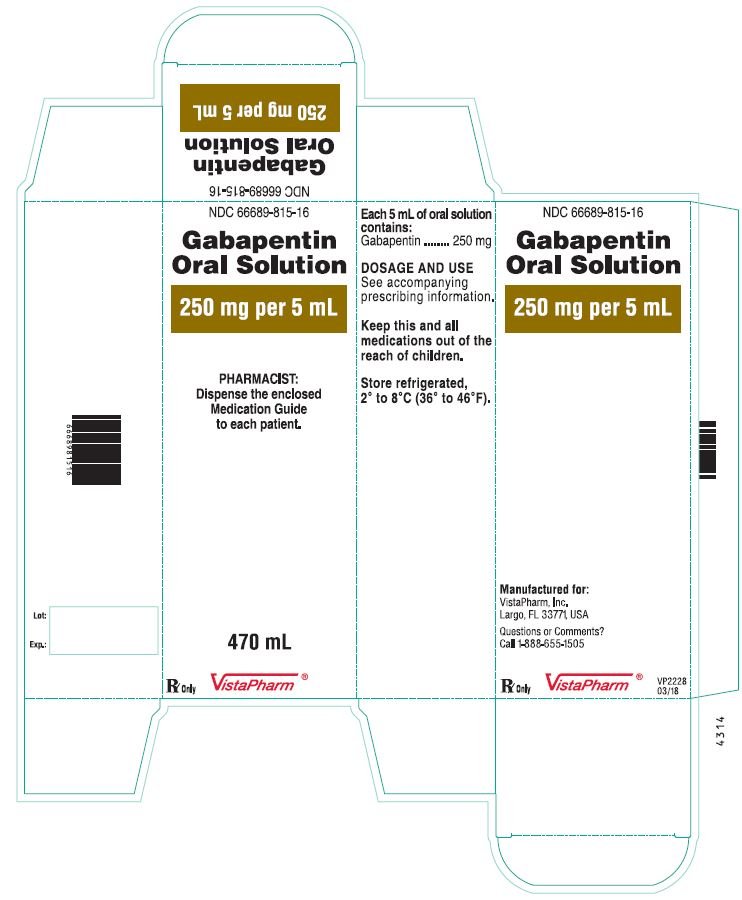
6.1 Beyond-use date and dating methods 23 6.1.1 General guidelines for assigning beyond-use dates 23 6.2 Master Formulation Record 24 6.2.1 Template for a Master Formulation Record 25 6.3 Ingredients used for non-sterile compounding — quality and storage 27 6.3.1 Selection of ingredients 27 6.3.2 Sources of ingredients 28 Generally, the expiration date of gabapentin after manufacture is usually 2 to 3 years. After dispensing by a pharmacy to the patient, the expiration date is generally set at 1 year (from the date of dispensing). There is no evidence to suggest that gabapentin turns harmful after it has expired. It has a shelf life of 5 years, and since the date on you med is different than the bottle that the pills were taking from—You drug should be good until 2019–. Is it still good to take? Gabapentin is available as oral capsules of 100 mg, 300 mg, and 400 mg; tablets of 100 mg, 300 mg, 400 mg, 600 mg, and 800 mg; and an oral solution of 50 mg/mL. However, the need often exists for a higher-concentration oral liquid, and if tablets or capsules are used as the drug source, a suspension will result. What are Beyond-Use Dates? Beyond-use dates (BUDs) are the date or time after which a compounded sterile preparation (CSP) or compounded nonsterile preparation (CNSP) may not be stored or transported and are calculated from the date or time of compounding. Why are Beyond-Use Dates Necessary? Each SolutionKitsTM – Gabapentin Compounding Kit contains: (i) 22.5 g of gabapentin powder USP; (ii) 440 mL of oral suspension vehicle, and (iii) 1 Plastic Funnel for Compounding. Using the kit and following the directions provided results in an oral suspension of 450mL containing 50 mg/mL of gabapentin oral solution. Contents NDC 46144-600-01 The simple, straightforward answer is: no, you should not use expired gabapentin. While it might be tempting to use leftover medication, especially if you’re in a pinch, using expired gabapentin comes with potential risks. I've heard that gabapentin has a shelf life of five years after expiration. Most pharmacists have to put a earlier expiration date on them however. And even if gabapentin is expired they pose no risk. (gabapentin) Oral Solution DESCRIPTION Neurontin ® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution (gabapentin) oral solution full prescribing information. Pfizer, NY, USA. 2015–09. Objectives: To investigate the stability and beyond-use date (BUD) of topical gabapentin in 3 commonly used Like all medications, Gabapentin has an expiration date, after which it may not be as effective or safe to use. But how long does Gabapentin actually last after its expiration date? According to experts, Gabapentin is typically safe to use for up to one year after its expiration date. Oral Liquid Formulations – Part XI INTRODUCTION With Part 11 of this series, we have reviewed and summarized 110 drugs in formulations with beyond- in Table 2 below, show that the solution is stable at both refrigeration and room temperatures for up to 3 use dates established and published in the peer reviewed literature. In fact, many medications, including gabapentin, are often safe and effective for a period of time beyond their expiration dates. The FDA conducted a study called the Shelf Life Extension Program (SLEP) to examine the stability of medications beyond their expiration dates. Can I Use Expired Gabapentin? Why You Shouldn’t Use Expired Gabapentin; Understanding Gabapentin Stability; Proper Disposal of Expired Gabapentin; Frequently Asked Questions (FAQs) about Expired Gabapentin. 1. Does gabapentin lose its effectiveness over time? 2. Can I use expired gabapentin for my dog? 3. **Yes, Gabapentin does expire.** Like most medications, gabapentin has an expiration date printed on its packaging or bottle. This date serves as a guideline for patients and healthcare professionals to use the drug safely and effectively. Neurontin® (gabapentin) Capsules, Neurontin® (gabapentin) Tablets, and Neurontin® (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The Conveniently packaged in a searchable format with quick access to associated formulas and validated stability-indicating studies conducted by third party institutions - our beyond-use-date (BUD) library provides reassurance on the stability of commonly prepared compounded medications including pediatric oral liquids, hormone creams, analgesic creams, hair care foams, and more. A beyond use date (BUD) is the date or time after which a compounded sterile preparation (CSP) or compounded nonsterile preparation (CNSP) may not be stored or transported and is calculated from the date or time of compounding. Pen should be discarded 30 days after initial use. 28 days after initial use. Protect from light. Do not use if solution is cloudy, colored, or contains solid particles. May be at room temp for up to 7 days prior to admin; After reconstitution, store at room temp & use within 6 hrs. A Beyond Use Date (BUD) for a compounded medication is similar to an expiration date given to any other drug. It is the date beyond which the use of the compounded medication is not recommended. In theory, it should be the date at which a compounded medication is no longer able to retain the same level of potency or stability it had on the day
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |