Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 | 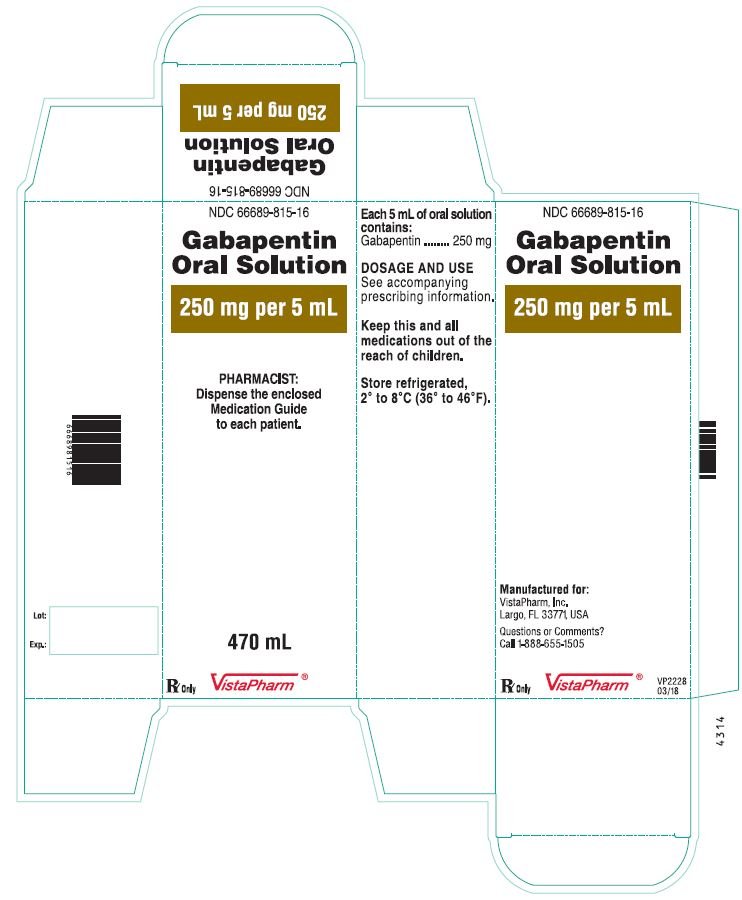 |
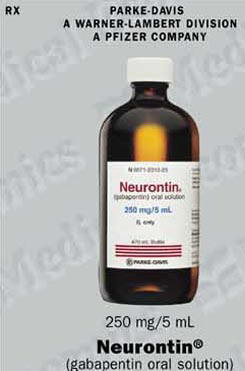
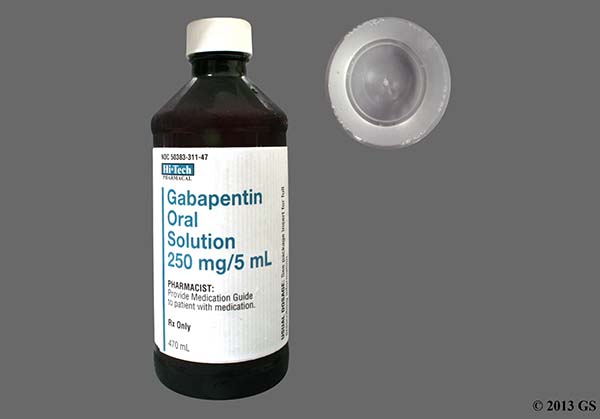
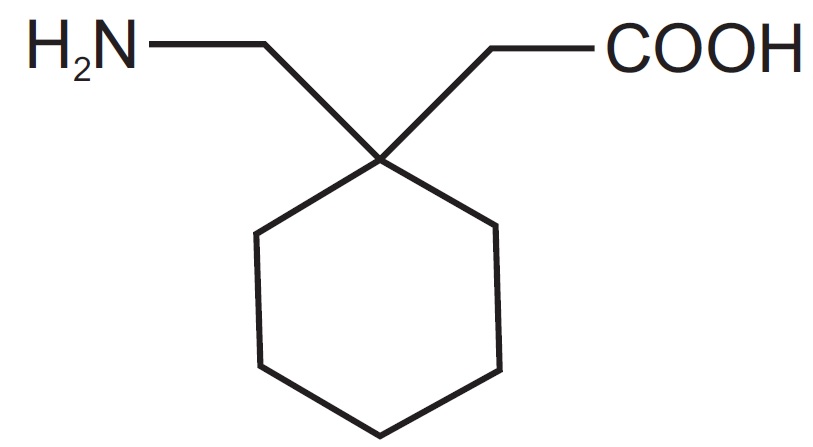
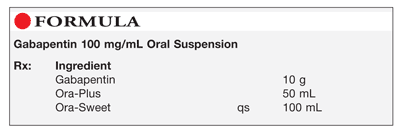
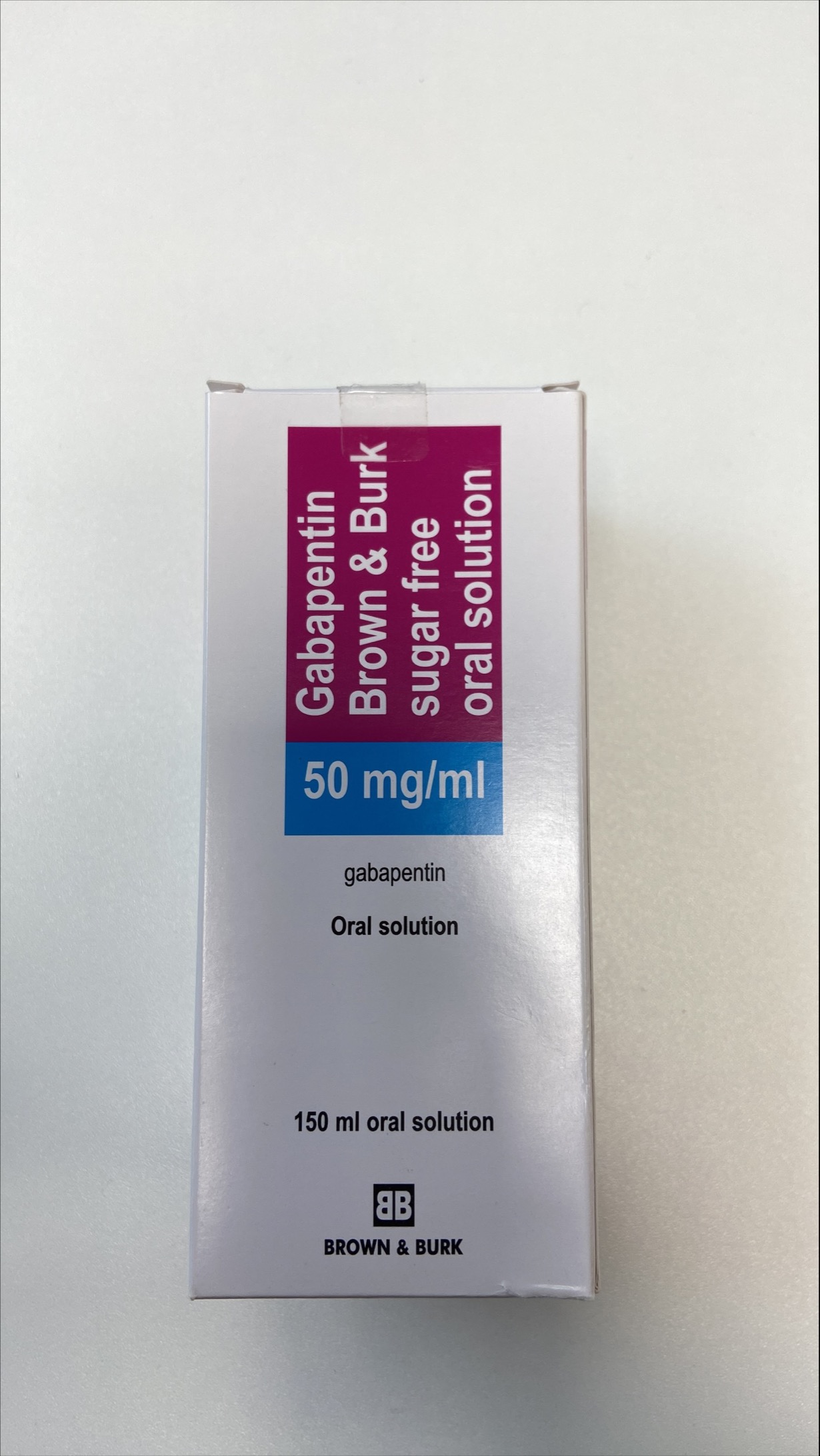
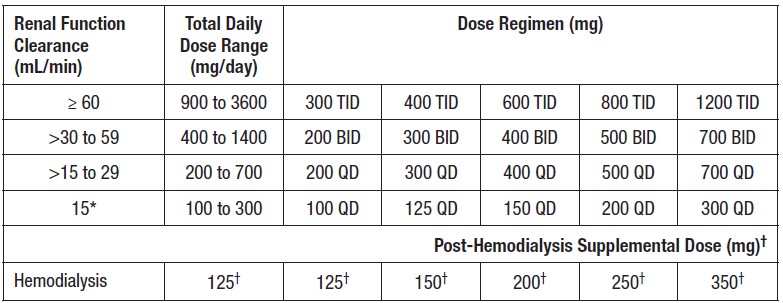
Each SolutionKitsTM – Gabapentin Compounding Kit contains: (i) 22.5 g of gabapentin powder USP; (ii) 440 mL of oral suspension vehicle, and (iii) 1 Plastic Funnel for Compounding. Using the kit and following the directions provided results in an oral suspension of 450mL containing 50 mg/mL of gabapentin oral solution. Contents NDC 46144-600-01 Material Name: Neurontin (gabapentin) Oral Solution Trade Name: NEURONTIN Chemical Family: Mixture Intended Use: Pharmaceutical product used as anticonvulsant 2. HAZARDS IDENTIFICATION Appearance: Clear, colorless liquid Statement of Hazard: Non-hazardous in accordance with international standards for workplace safety. Additional Hazard Material Name: Neurontin (gabapentin) Oral Solution Trade Name: NEURONTIN Chemical Family: Mixture Relevant Identified Uses of the Substance or Mixture and Uses Advised Against Intended Use: Pharmaceutical product used as anticonvulsant Details of the Supplier of the Safety Data Sheet 2. HAZARDS IDENTIFICATION Classification of the Substance or Neurontin (gabapentin) Oral Solution Special Notice: Our database is made up of both MSDS and SDS. Carefully review the (M)SDS below to see if it’s the version you're looking for. Material Safety Data Sheet . Material Name: Gabapentin Oral Solution, 250 mg / 5 mL _____ Page 2 of 5 Issue Date: 02/16/12 Revision: 1.0000 Print Date: 2/16/2012 . First Aid: Inhalation. Remove the person from the exposed area to fresh air immediately. Revision date: 02-Jan-2007 Page 1 of 6 MATERIAL SAFETY DATA SHEET Version: 1.2 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Material Name: Gabapentin Capsules (100 mg, 300 mg and 400 mg) Rosemont Pharmaceuticals Limited Registered office: Rosemont House Yorkdale Industrial Park Braithwaite Street Leeds LS11 9XE. Registered No. 924648 VAT number: GB 351092620 Main switchboard Chemical Name Gabapentin-d10 Catalogue # G117254 Company Toronto Research Chemicals 2 Brisbane Road Toronto, ON M3J 2J8 CANADA Telephone FAX Email +14166659696 +14166654439 orders.trc@lgcgroup.com Product Uses To be used only for scientific research and development. Not for use in humans or animals. These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 INDICATIONS AND USAGE Gabapentin oral solution is indicated for: • Postherpetic neuralgia in adults ( 1) 52 Week(s) Monkey Oral 250 mg/kg/day NOAEL None identified Rat Oral LD50 100 g/kg Gabapentin 13 Week(s) Mouse Oral 1000 mg/kg/day NOAEL No effects at maximum dose 2 Year(s) Mouse Oral, in feed 2000 mg/kg/day NOEL Not carcinogenic Rat Inhalation LC50 > 2000 mg/m3 · Trade name:Gabapentin · Synonym CI-945 1-(aminomethyl)-cyclohexaneacetic acid · Article number:10008346 · CAS Number: 60142-96-3 · EC number: 262-076-3 · Application of the substance / the mixture This product is for research use - Not for human or veterinary diagnostic or therapeutic use. · Details of the supplier of the safety data sheet SDS: Gabapentin Oral Solution 4 of 7 Respiratory Protection: Where respirators are deemed necessary to reduce or control occupational exposures, use NIOSH-approved respiratory protection and have an effective respirator program in place (applicable U.S. regulation OSHA 29 CFR 1910.134). Gabapentin Rat Subcutaneous LD50 > 4000mg/kg Material Name: Gabapentin Tablets (Neurontin) Gabapentin Eye Irritation Rabbit Non-irritating Povidone Mouse Oral LD50 > 5000 mg/kg Gabapentin Rat Oral LD50 100 g/kg Version: 2.1 52 Week(s) Rat Oral250 mg/kg/day NOAEL Liver, Kidney Product name : Gabapentin CBnumber : CB3263316 CAS : 60142-96-3 EINECS Number : 262-076-3 Synonyms : gabapentin,gabapentine Relevant identified uses : For R&D use only. Not for medicinal, household or other use. Uses advised against : none Company : Chemicalbook Address : Building 1, Huihuang International, Shangdi 10th Street, Haidian District Show this safety data sheet to the doctor in attendance. Immediate medical attention is required. Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: carboxymethylcellulose sodium, methylparaben, propylene glycol, propylparaben, purified water, xylitol, anise flavor, artificial strawberry flavor and hydrochloric acid added for adjustment of pH. Material Safety Data Sheet . Material Name: Gabapentin Oral Solution, 250 mg / 5 mL _____ Page 5 of 5 Issue Date: 02/16/12 Revision: 1.0000 Print Date: 2/16/2012 . Additional Regulatory Information. Component Analysis - Inventory. Component CAS # TSCA CAN EEC Water 7732-18-5 Yes DSL EINECS Gabapentin 60142-96-3 No No EINECS SDS: Gabapentin Oral Solution 1 of 7 SAFETY DATA SHEET 1. Identification Product Identifier: Gabapentin Oral Solution Synonyms: Cyclohexaneacetic acid, 1-(aminomethyl)- National Drug Code (NDC): 50383-311-07 50383-311-09 50383-311-47 Recommended Use: Pharmaceutical. Company: Akorn, Inc. 1925 West Field Court, Suite 300 MATERIAL SAFETY DATA SHEET Version: 1.3 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Material Name: Neurontin (gabapentin) Oral Solution Trade Name: NEURONTIN(R) Chemical Family: Mixture Intended Use: Pharmaceutical product used as anticonvulsant 2. COMPOSITION/INFORMATION ON INGREDIENTS Hazardous
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |