Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
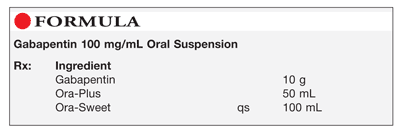
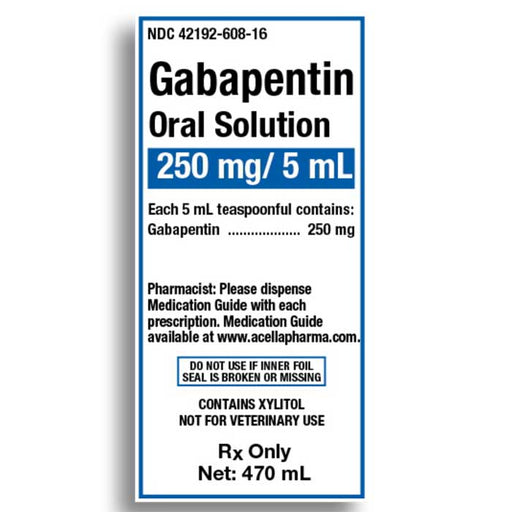
The solution stability of gabapentin in buffered systems was studied in order to facilitate the formulation of a liquid product. The degradation of the drug was followed as a function of pH, buffer concentration, ionic strength, and temperature. (gabapentin) oral solution full prescribing information. Pfizer, NY, USA. 2015–09. The stability of gabapentin pediatric oral suspension prepared from commercially available 400 mg capsules Stopping gabapentin oral solution suddenly can cause serious problems. Gabapentin oral solution can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin oral solution may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Compounded preparations of gabapentin 100 mg/mL prepared using Oral Mix or Oral Mix SF, bulk drug powder or capsules and packaged in amber PET bottles or amber oral syringes remained stable for at least 90 days at 25°C. Gabapentin is not available in a liquid dosage form for clinical use. This study was designed to develop two oral gabapentin suspensions and determine their stability under refrigeration or at room temperature. Commercially available gabapentin capsules were used to prepare two suspensions: one in e The recommended maintenance dose of gabapentin oral solution in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin oral solution may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF suspending vehicles. Suspensions were packaged in amber plastic bottles and amber plastic syringes at 25°C / 60%RH for up to 9 Gabapentin Focus is an oral solution at a concentration of 50 mg gabapentin per ml. Thus, the amount dosed (900 to 3600 mg per day divided into 2 or 3 dosages) depends on the amount solution dosed. Therefore, the product fulfils point a to c. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are See full prescribing information for GABAPENTIN. Gabapentin capsules, for oral use Gabapentin tablets, for oral use Gabapentin oral solution Initial U.S. Approval: 1993 INDICATIONS AND USAGE Gabapentin is indicated for: • • DOSAGE AND ADMINISTRATION • • • DOSAGE FORMS AND STRENGTHS • • • CONTRAINDICATIONS Known hypersensitivity Stability: A beyond-use date of up to 56 days at room temperature or 91 days at refrigerated temperature may be used for this preparation. 1,2 Quality Control: Quality-control assessment may include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability Do not stop taking Gabapentin Oral Solution without first talking to your healthcare provider. Stopping Gabapentin Oral Solution suddenly can cause serious problems. Gabapentin Oral Solution can cause serious side effects including: 1. Like other antiepileptic drugs, Gabapentin Oral Solution may cause suicidal thoughts or actions Gabapentin oral solution is stable at room temperature for up to 24 hours, according to the American Society of Health-System Pharmacists. After this time frame, it begins to degrade and should be discarded and replaced with a fresh dose. SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) Friciu M, Roullin VG, Leclair G. Stability of gabapentin in extemporaneously compounded oral suspensions. PLoS ONE. 2017; 12(4): 1-11. Disclaimer: The information in this compounding and preparation worksheet was developed for in-house use only by The Hospital for Sick Children (“SickKids”) and its staff. Gabapentin (C9H17NO2, MW 171.24) occurs as white to off-white, crystalline solid that is freely soluble in water. Gabapentin 100 mg/mL oral suspension was prepared using a 1:1 mixture of ORA-Plus®/ORA-Sweet® and stored at both room and refrigerated temperatures. This study reports the stability of extemporaneously prepared gabapentin oral suspensions prepared at 100 mg/mL from bulk drug and capsules in either Oral Mix or Oral Mix SF sus- pending vehicles. How should I store Gabapentin Oral Solution? •Store Gabapentin Oral Solution in the refrigerator between 36°F to 46°F (2°C to 8°C). Keep gabapentin and all medicines out of the reach of children. General information about the safe and effective use of gabapentin Gabapentin oral suspension (Neurontin) Refrigerate at 36º to 46º F (2º to 8ºC). Contact manufacturer.*** Off-label § information indicates oral solution stable for seven days at temps up to 86 o F (30 o C). 6 Pfizer 800-438-1935 Glatiramer acetate injection (Copaxone) Refrigerate at 36º to 46º F (2º to 8ºC). One month at room Lyrica oral solution, 20 mg/mL is a new oral formulation of pregabalin (currently approved as a hard capsule dosage form for oral use (25 to 300 mg) designed to be given orally with or without food. The referenced approved NDA # is 21-466. The oral solution concentration was selected at 20 mg/mL for dosing flexibility. The daily dose
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |