Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
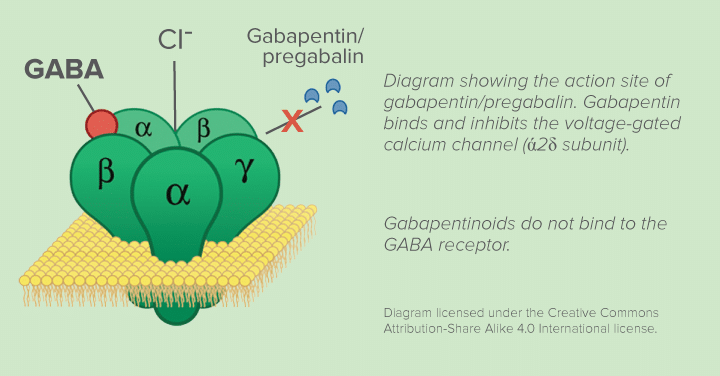
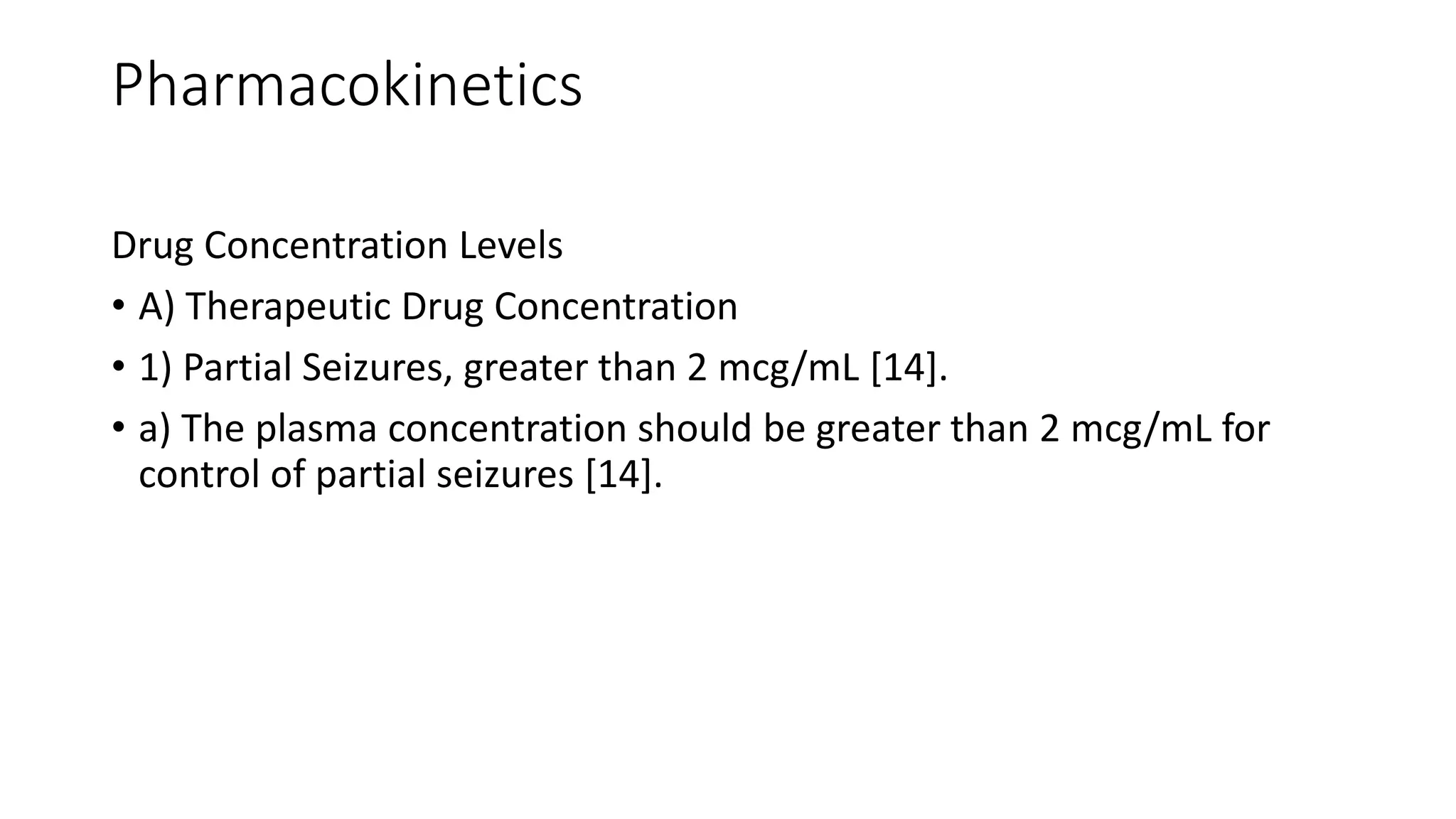
The mechanism by which Gabapentin exerts its analgesic action is unknown, but in animal models of analgesia, Gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). Includes Gabapentin indications, dosage/administration, pharmacology, mechanism/onset/duration of action, half-life, dosage forms, interactions, warnings, adverse reactions, off-label uses and more. While gabapentin's mechanism of action is generally understood, it appears to be a pharmacologic option for treating issues involving the gamma-aminobutyric acid (GABA) receptor system. Gabapentin is a relatively safe, readily available, and effective drug for alcohol-use disorder treatment, specifically for the abstinence maintenance phase. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence Gabapentin, like other gabapentinoid drugs, acts by decreasing activity of the α 2 δ-1 protein, coded by the CACNA2D1 gene, first known as an auxiliary subunit of voltage gated calcium channels. [13][14][15] However, see Pharmacodynamics, below. Gabapentin, per its namesake, was designed to be a GABA analog. Further studies revealed that it does not in fact bind to GABA receptors, but primarily works by another mechanism of action which remains to be elucidated. It is important to remember that Gabapentin does NOT bind to GABA receptors. Mechanism of action: By inhibiting the voltage-gated calcium channels in the CNS, gabapentin reduces the release of excitatory neurotransmitters (mostly noradrenaline, dopamine and serotonin), and therefore decreases epileptogenesis. Clinical effects In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). GABAPENTIN- gabapentin capsule Major Pharmaceuticals-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN CAPSULES safely and effectively. See full prescribing information for GABAPENTIN CAPSULES. GABAPENTIN capsules, for oral use Initial U.S. Approval: 1993 INDICATIONS AND USAGE Visit DrugBank.com for detailed drug and target data for your pharmaceutical Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm The new antiepileptic medications are prescribed for the treatment of patients with seizure disorders since 17 years ago. Gabapentin (GBP) was approved on January 1994 as adjunctive treatment in patients 12 years or older with partial seizures, with or devoid of secondary generalization. Neurontin; Ther. Class. analgesics. anticonvulsants. mood stabilizers. Pharm. Class. gamma aminobutyric acid gaba analogues. Controlled Substance Schedule: V(only schedule V in some states) There's more to see -- the rest of this topic is available only to subscribers. Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. The chemical structure of gabapentin (Neurontin) is derived by addition of a cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin prevents seizures in a wide variety of models in animals, including generalized tonic-clonic and partial seizures. Gabapentin has no activity at Gabapentin is available in two extended-release formulations in addition to the immediate release: a gastric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neuralgia. Mechanism of Action: Moore RA et al (2014): Gabapentin for chronic neuropathic pain and fibromyalgia in adults. The Cochrane database of systematic reviews 4 Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific bindin Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |