Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 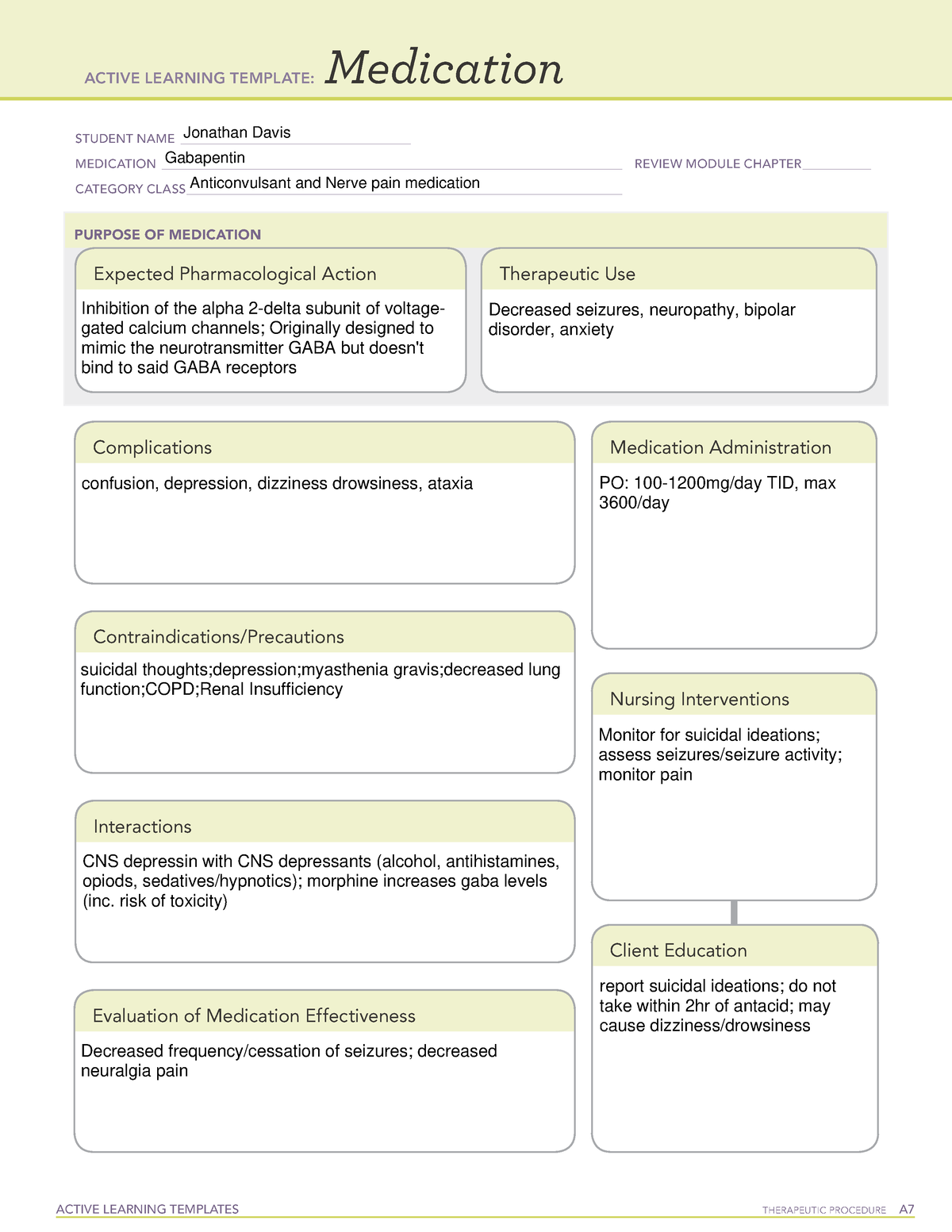 |
Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin | C9H17NO2 | CID 3446 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Gabapentin is in a class of medications called anticonvulsants. What are the brand names of gabapentin? Gabapentin is available as both a brand name product and a generic product (chemically the same, usually lower cost than the brand name product). Brand names of gabapentin include Horizant®, Gralise® and Neurontin®. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Instruct patient to take medication exactly as directed. Patients on 3 times daily dosing should not exceed 12 hr between doses. Take missed doses as soon as possible; if less than 2 hr until next dose, take dose immediately and take next dose 1-2 hr later, then resume regular dosing schedule. While gabapentin's mechanism of action is generally understood, it appears to be a pharmacologic option for treating issues involving the gamma-aminobutyric acid (GABA) receptor system. Gabapentin is a relatively safe, readily available, and effective drug for alcohol-use disorder treatment, specifically for the abstinence maintenance phase. The gabapentinoids are often recommended as first-line treatments for the management of neuropathic pain. The differing pharmacodynamic and pharmacokinetic profiles can have implications for clinical practice. This article has summarised these key differences. In addition to their use in managing ne Gabapentin, under the brand name Neurontin, was first approved in May 1993 for the treatment of epilepsy in the United Kingdom, and was marketed in the United States in 1994. [43] [44] Subsequently, gabapentin was approved in the United States for the treatment of postherpetic neuralgia in May 2002. [45] Gabapentin is a medication commonly prescribed to treat various conditions, including epilepsy, neuropathic pain, and restless legs syndrome. This guide aims to educate patients about important considerations, including dosage instructions, potential side effects, and precautions, to ensure safe and effective use of gabapentin. Gabapentin in the management of restless legs syndrome (RLS) has been evaluated in small controlled trials, demonstrating benefits compared with placebo. Gabapentin enacarbil is FDA-approved for the treatment of RLS Garcia-Borreguero 2002, Saletu 2010. The . Social anxiety disorder, adjunct to antidepressants or monotherapy (alternative agent)c Gabapentin: Class: Analgesics Chemistry: Structural analogue of GABA Routes of administration: Oral only. Absorption: Variable oral bioavailability; 60% in small doses, 40% in large doses, mainly because it is absorbed by saturable facilitated transport (L-amino acid transporter) Solubility Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Gabapentin is in a class of medications called anticonvulsants. Gabapentin treats seizures by decreasing abnormal excitement in the brain. Gabapentin relieves the pain of PHN by changing the way the body senses pain. It is not known exactly how gabapentin works to treat restless legs syndrome. How should this medicine be used? Gabapentin [1- (aminomethyl)cyclohexane acetic acid] is␣a␣novel anti-epileptic agent, originally developed as a gamma-aminobutyric acid (GABA)-mimetic compound to treat spasticity, and has been shown to have potent anticonvulsive effects [1, 2]. All pharmacological actions following gabapentin administration are due to the activity of the parent compound; gabapentin is not appreciably metabolized in humans. Oral Bioavailability Gabapentin bioavailability is not dose proportional; i.e., as dose is increased, bioavailability decreases. Although it has been known for some time that gabapentin must bind to the α 2 δ-1 protein in order to act pharmacologically (see Pharmacodynamics), the three-dimensional structure of the α 2 δ-1 protein with gabapentin bound (or alternatively, the native amino acid, L-Isoleucine bound) has only recently been obtained by cryo-electron Drug Class: Antiepileptic Basic & Clinical Pharmacology, 13th Ed. (2014): Gabapentin for chronic neuropathic pain and fibromyalgia in adults. The Cochrane Gabapentin is available in two extended-release formulations in addition to the immediate release: a gastric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neuralgia. All pharmacological actions following gabapentin administration are due to the activity of the parent compound; gabapentin is not appreciably metabolized in humans. Oral Bioavailability. Gabapentin bioavailability is not dose proportional; i.e., as dose is increased, bioavailability decreases. Horizant® (gabapentin enacarbil) Therapeutic class: Anti-epileptic, Anticonvulsant; Pharmacologic class: 1-amino-methyl cyclohexoneacetic acid, Gamma-aminobutyric acid (GABA) analogue; FDA Approved: December 30, 1993; Chemical Formula: C9H17NO2; Pregnancy Category: C; Habit forming? No
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |