Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
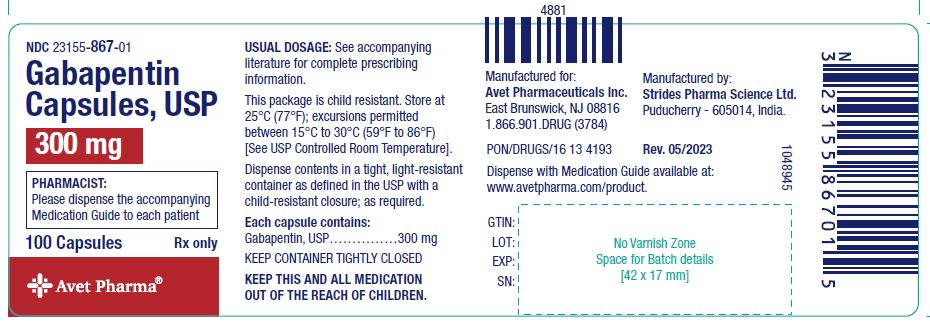
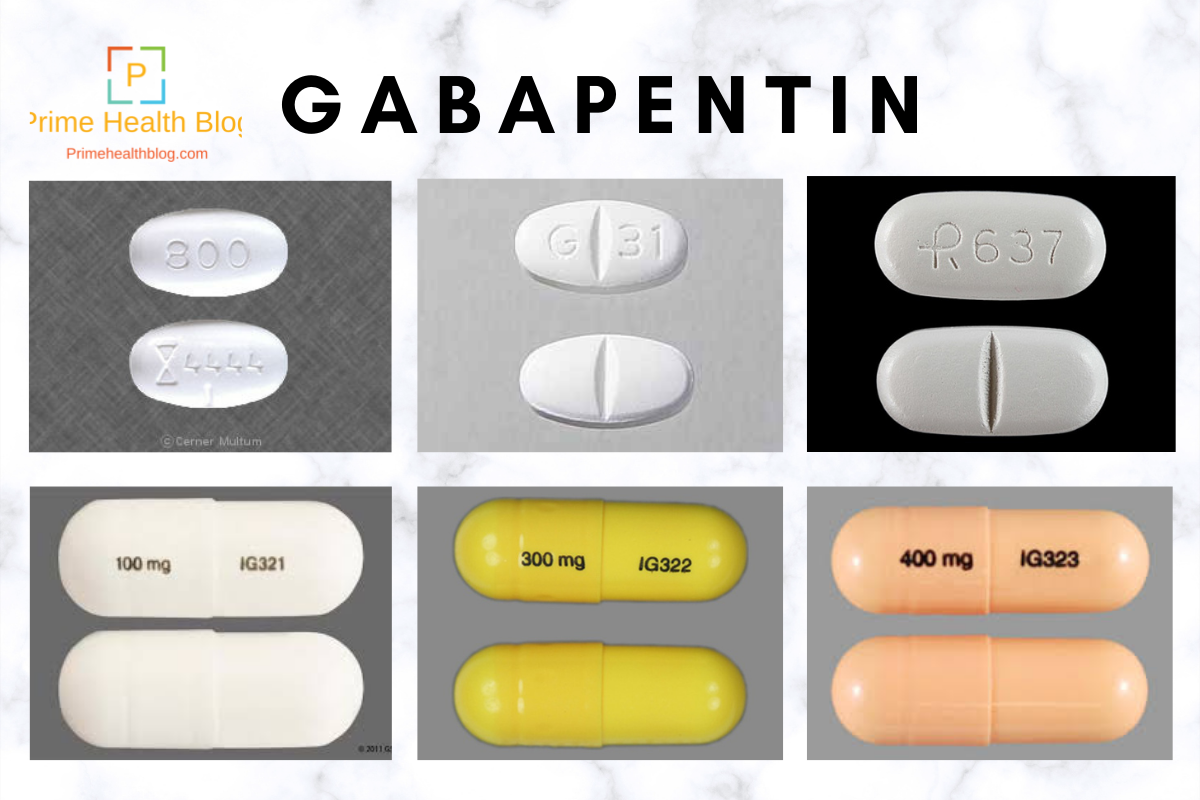
Four unpublished studies met inclusion criteria; in three, participants had pain following dental surgery, and one followed major orthopaedic surgery; 177 participants were treated with a single dose of gabapentin 250 mg, 21 with gabapentin 500 mg, and 172 with placebo. They found that a single preoperative dose of 1200 mg effectively reduced postoperative pain and opioid consumption. Multiple doses of gabapentin preoperatively, and continued postoperatively, did not appear to reduce pain scores. Incidence of opioid adverse effects such as vomiting, and pruritus were lower in the gabapentin group. pain assessment in cats and dogs, as well as an initial therapy plan with guidance on reassessing and adjusting the plan as needed. As such, these guidelines discuss pain management as a therapeutic con-tinuum consisting of assessment, treatment, reassessment, and plan revision. Pain management has been recognized as an essential compo- Randomised placebo-controlled trials (RCTs) investigating peri-operative gabapentin for post-operative pain management were eligible for inclusion. Eligible outcomes were relevant pain outcomes such as pain scores, time to first analgesic request and post-operative cumulative opioid consumption. Avoid pre-operative gabapentin doses >600 mg due to increased risk of side effects • Level 3 Scheduled gabapentin doses should be avoided in the post-operative period unless otherwise indicated for neuropathic pain Initial gabapentin doses for post-operative neuropathic pain should be limited to 300 mg per 24 hours Wean gabapentin over at Gabapentin is commonly indicated in the treatment of seizures. 27 Gabapentin, which acts on the nociceptive processes involved in central sensitization, has been shown to reduce hypersensitivity associated with nerve injury (hyperalgesia) and postoperative pain and inflammation in animal models. 28 Interestingly, gabapentin’s antiemetic In this trustworthy systematic review, use of gabapentin for post-operative pain management was scrutinized. In summary, the quality of evidence for a clinically relevant benefit of gabapentin is low, and, importantly, harm may be present. Gabapentin caused a 35% reduction in total opioid consumption over the first 24 h following surgery (ratio of means 0.65, 95% CI 0.59 to 0.72), a significant reduction in postoperative pain at rest (in the first 24 h) and with movement (at 2 h, 4 h and 12 h), regardless of whether treatment effects were expressed as ratios of means or weighted m In the analysis of gabapentin as add-on analgesic to another non-opioid analgesic regimen found a mean reduction in 24-h morphine consumption of 1.2 mg [-0.3, 2.6; TSA-adjusted CI: -0.3, 2.6] in trials with low risk of bias. The purpose of this review is to critically appraise the evidence for the use of gabapentinoids for acute pain management and its impact on the development of chronic pain after surgery. Recent findings: Recent meta-analyses have revealed that prior data likely have overestimated the beneficial effects of gabapentinoids in acute perioperative Post-operative gabapentin (600 mg) may be equally effective as a preoperative dose in decreasing PACU narcotic use. Concomitant administration of gabapentin as part of a multi-modal pain management strategy including narcotics, non-steroidal anti-inflammatory drugs (NSAIDS), and muscle relaxants does not improve pain scores. 300 mg on day 1, 600 mg on day 2, and 900 mg from day 3 to day 30 after surgery: Acute post-operative pain intensity in gabapentin group was significantly lower than the placebo group p<0.05). The rate of PLP at the last follow-up was lower in the gabapentin group (43.48%) compared to placebo (77.27%, p=0.033). Hah et al., 2018 25 Pain and sedation scores (median and lower-upper range) measured prior to surgery (ie, baseline) and at 0.5, 1, 2, 4, 6, 8, 12, 18, 24, 32, 40, 48, 56, 64 and 72 hr after tracheal extubation in dogs undergoing mastectomy treated with gabapentin (Gabapentin) or placebo (Placebo). The coprimary outcomes were postoperative acute pain at 6, 12, 24, 48, and 72 h after surgery measured by any quanti-tative pain scale.58 Secondary outcomes were postoperative subacute pain (defined as pain intensity during postopera-tive weeks 4 to 12); incidence of postoperative chronic pain During the last 15 years, gabapentin has become an established component of postoperative pain treatment. Gabapentin has been employed in a wide range of doses, but little is known about the optimal dose, providing the best balance between benefit and harm. Perioperative gabapentin, 1200 mg, administered preoperatively plus 600 mg every 8 hours continued for 72 hours after surgery did not affect time to pain cessation, the rate of pain resolution, or the proportion of patients with chronic pain at 6 months or 1 year following surgery. They used a gabapentin dose of 1.2 g per day treatment 1 hour before surgery and for 2 days after surgery and investigated its effect on postoperative acute pain. Some randomized controlled studies have carried out to evaluate the effects of gabapentin on pain relief after TKA. However, no solid result was made about it. The purpose of this Meta-Analysis of Randomized Controlled Trials (RCTs) was to estimate the overall effect of pain control of gabapentin versus placebo after a TKA. Prescription Post‐operative celecoxib 400 mg initial dose followed by 200 mg bid for 5 days is recommended in patients having a colorectal resection where NO anastomosis is performed (for example, abdominal perineal resection) and where no contraindications to its use are present.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |