Gallery
Photos from events, contest for the best costume, videos from master classes.
00110-0/asset/52dc3cdc-6865-49eb-827f-f6defd446616/main.assets/gr1c.gif) |  |
 |  |
 | |
 |  |
 |  |
 |  |
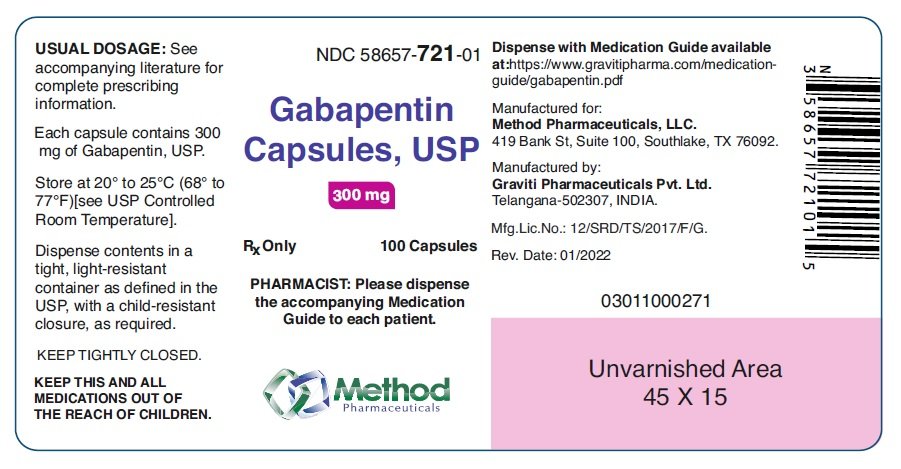
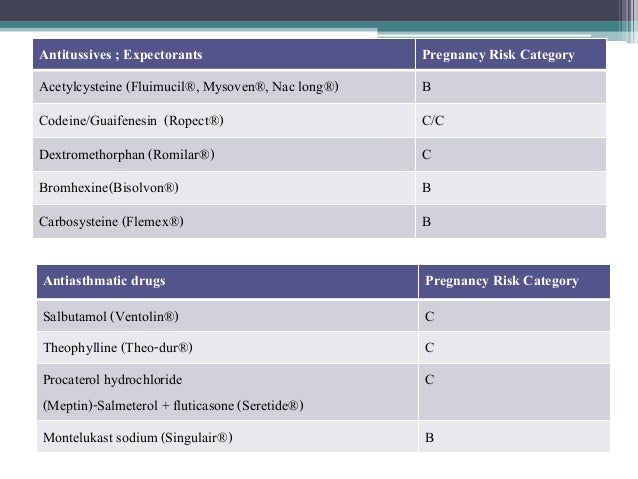
Abstract Background. Despite the widespread use, only sparse information is available on the safety of gabapentin during pregnancy. We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes. Pregnancy Category C: Gabapentin has been shown to be fetotoxic in rodents, causing delayed ossification of several bones in the skull, vertebrae, forelimbs, and hindlimbs. These effects occurred when pregnant mice received oral doses of 1000 or 3000 mg/kg/day during the period of organogenesis, or approximately 1 to 4 times the maximum dose of Advice and warnings for the use of Gabapentin during pregnancy. FDA Pregnancy Category C - Risk cannot be ruled out Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. It is not known if gabapentin can make it harder to get pregnant. Sexual dysfunction (including loss of desire to have sex and loss of ability to have an orgasm) has been reported among women who take gabapentin. นิยามศัพท์. Pregnancy Category. A: จากการศึกษาไม่พบความเสี่ยงต่อทารกในครรภ์ทั้งในไตรมาส 1และ 3 ยาที่จัดอยู่ในระดับนี้แทบไม่มีอันตรายต่อทารกในครรภ์ Keywords:Gabapentin;Pregnancy;Epilepsy;Teratogenicity 1. Introduction levetiracetam, have been classified as category C drugs for pregnancy [7–13]. Animal The U.S. Food and Drug Administration classifies gabapentin (Neurontin) as a Pregnancy Category C medication, which means that animal studies conducted on this medication has caused harm on the fetus. We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. Gabapentin has been assigned to pregnancy category C. Animal studies have revealed evidence of fetotoxicity involving delayed ossification in several bones of the skull, vertebrae, forelimbs and hindlimbs. Hydroureter and hydronephrosis have also been reported in animal studies. There are no controlled data in human pregnancy. The objective of this study was to assess the safety of gabapentin (Neurontin) exposure in human pregnancy. Prospective and retrospective data concerning 51 fetuses, including 3 twin gestations, were collected from 39 women with epilepsy and other disorders exposed to gabapentin during pregnancy. It’s important to know the pregnancy category of any medications you may be taking. Gabapentin, a medication commonly used to treat epilepsy and neuropathic pain, falls into category C for pregnancy. Category C means that animal studies have shown adverse effects on the fetus, but there are no well-controlled and adequate studies in humans. Overview of the five pregnancy risk categories, established by the FDA to indicate the potential of a drug to cause birth defects if used during pregnancy. Gabapentin is a pregnancy category C, which means risk cannot be ruled out. Gabapentin can be transmitted through human milk. It’s currently unknown how breast milk containing gabapentin affects newborns or even the milk production process. Consult your physician about breastfeeding while taking gabapentin. Does gabapentin affect female fertility? gabapentin (gab ah pen' tin)Neurontin . Pregnancy Category C . Drug class. Antiepileptic . Therapeutic actions. Mechanism of action not understood; antiepileptic activity may be related to its ability to inhibit polysynaptic responses and block posttetanic potentiation. This article summarizes the current literature regarding gabapentin use during pregnancy and related prenatal and neonatal exposure outcomes with special consideration for interactions between gabapentin and opioid use. Pregnancy category C, safety unknown in breastfeeding. $55 ($145) Gabapentin (Neurontin) No. Variable. Studies have used 300 mg twice per day or once-daily dosages up to 1,800 mg at bedtime. Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
00110-0/asset/52dc3cdc-6865-49eb-827f-f6defd446616/main.assets/gr1c.gif) |  |
 |  |
 | |
 |  |
 |  |
 |  |