Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
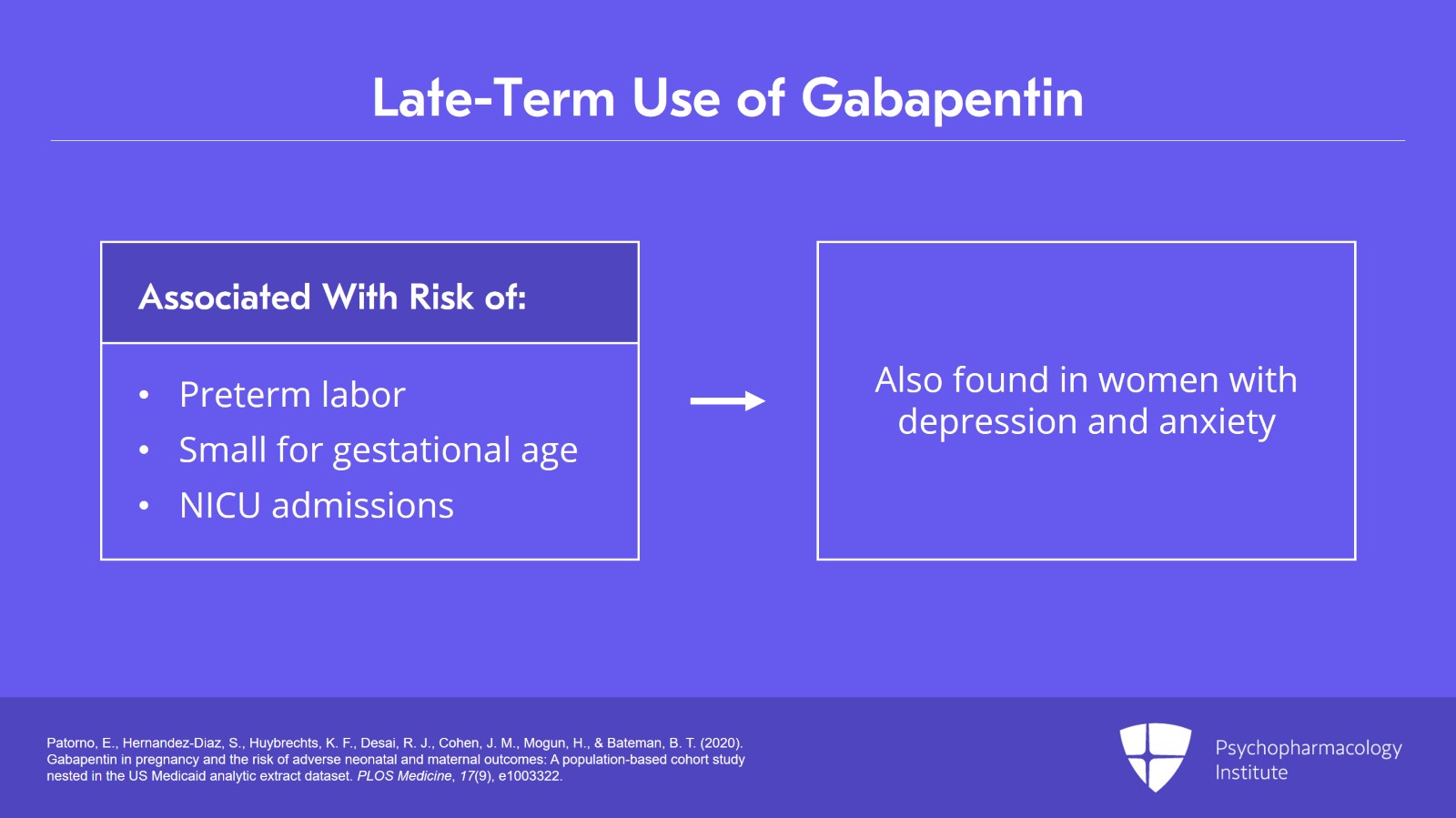
To provide information regarding the effects of in utero exposure to gabapentin, physicians are advised to recommend that pregnant patients taking gabapentin enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. FDA Pregnancy Categories FDA Pregnancy Risk Information: An Update. In 2015 the FDA replaced the former pregnancy risk letter categories on prescription and biological drug labeling with new information to make them more meaningful to both patients and healthcare providers. The FDA received comments that the old five-letter system left patients NEURONTIN is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan Advice and warnings for the use of Gabapentin during pregnancy. FDA Pregnancy Category C - Risk cannot be ruled out Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. Pregnancy Registry: If you become pregnant while taking gabapentin tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as gabapentin, during pregnancy. Encourage women who are taking gabapentin during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling the toll-free number 1-888-233-2334 or FDA has published a rule that provides a framework for clearly communicating information on the benefits and risks of using a drug during pregnancy and lactation. Neurontin (gabapentin) is used to treat pain you may have from shingles (postherpetic nerve pain). It is also used with other seizure medicines for partial onset seizures in patients 3 years and older. Gralise (gabapentin) is only used for pain after having shingles (postherpetic nerve pain). It should not be used for any other medical condition. We have data on 223 pregnancy outcomes exposed to gabapentin and 223 unexposed pregnancies. The rates of major malformations were similar in both groups (p = 0.845). There was a higher rate of preterm births (p = 0.019) and low birth weight <2,500 g (p = 0.033) in the gabapentin group. gabapentin dose may be required in patients who have age related compromised renal function. (See PRECAUTIONS, Geriatric Use, and DOSAGE AND ADMINISTRATION.) Pediatric: In addition to being currently US Food and Drug Administration (FDA)-approved for the treatment of partial seizures and postherpetic neuralgia, gabapentin is extensively used off-label for many conditions, including neuropathic pain, fibromyalgia, anxiety, and tremor. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or 5.3 Withdrawal of Gabapentin 5.4 . Tumorigenic Potential 5.5 . Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity 5.6. Laboratory Tests 6 ADVERSE REACTIONS . 6.1 Clinical Trials Experience . 6.2 Postmarketing and Other Experience with other Formulations of Gabapentin . 7 DRUG INTERACTIONS . 7.1 Phenytoin We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes. Methods and findings: Using the United States Medicaid Analytic eXtract (MAX) dataset, we conducted a population-based study of 1,753,865 Medicaid-eligible pregnancies between January 2000 and December 2013. Animal studies have shown that gabapentin intake during pregnancy increases the risk of embryo fetal toxicity, specifically on mice. For rats, adverse effects on offspring development were common among those who had gabapentin during pregnancy. Talk to your doctor about taking gabapentin if you are pregnant or are planning on being pregnant. There was an increased risk of preterm birth among women exposed to gabapentin either late (RR=1.28 [CI 1.08-1.52], p < 0.01) or both early and late in pregnancy (RR=1.22 [1.09-1.36], p < 0.001). 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |