Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 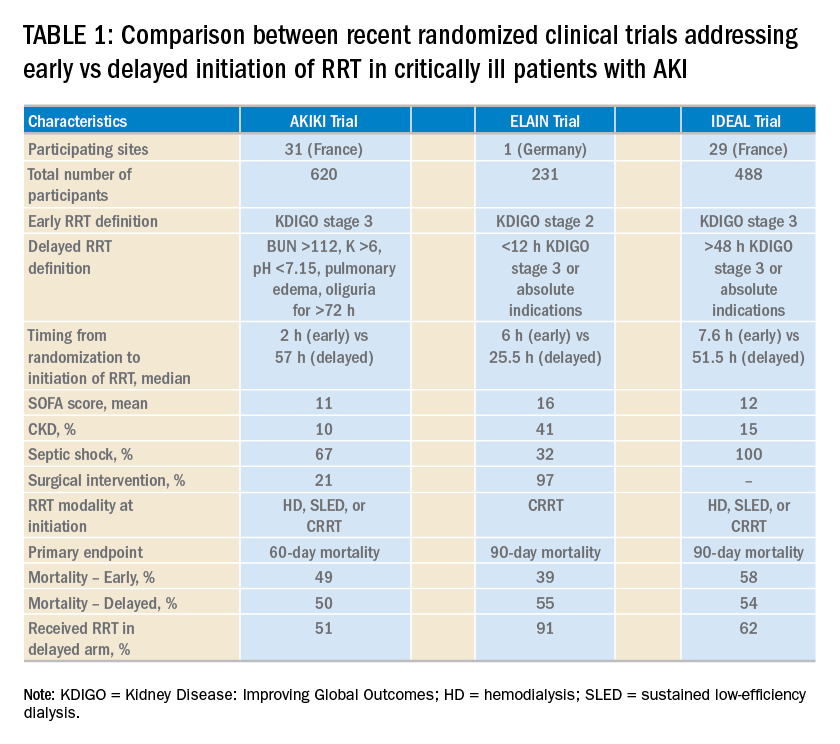 |
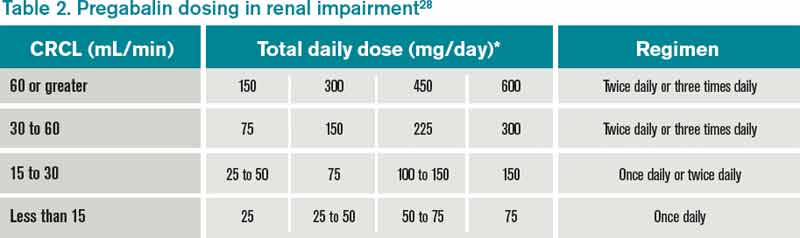
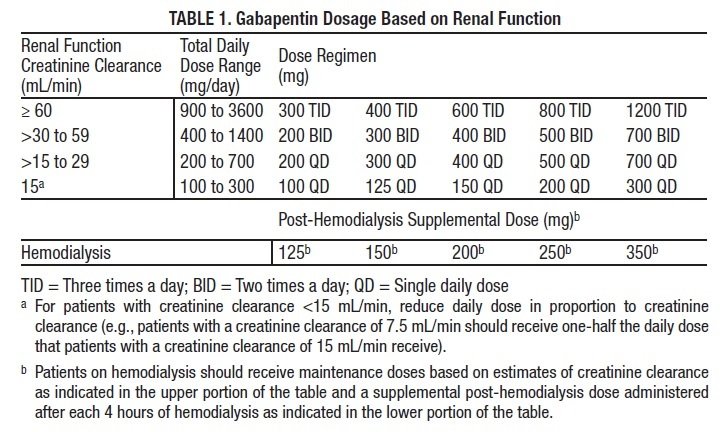
Continuous renal replacement therapy (CRRT) is used in approximately 5% to 10% of patients with AKI, and affects medication clearance.4 The optimal intensity of renal replacement therapy in critical illness is debated, but a prospective, multi-center, randomized trial demonstrated no significant impact of higher-intensity renal replacement therapy Objectives: The aim of this review was to evaluate current literature for dosing recommendations for the use of antiepileptic medications in patients receiving renal replacement therapy (RRT). Data sources: With the assistance of an experienced medical librarian specialized in pharmacy and toxicology, we searched MEDLINE, EMBASE, CINAHL, Web of dialytic clearance of gabapentin was reported to be 90% of the CL cr and 75% of the urea clearance, 10 corresponding to a serum elimination half-life dur-ing HD of 3.8 hours. Gabapentin also appears to be reasonably cleared using continuous renal replacement therapy, with the measured gabapentin clearance w80% of the measured urea clearance.11 In a study on 21 hemodialysis patients (10 females and 11 males with mean age of 58 years), we found that gabapentin at a dose of 100 mg is a safe effective therapy for restless legs syndrome. It can significantly reduce the intensity of restless legs syndrome among hemodialysis patients. Gabapentin also appears to be reasonably cleared using continuous renal replacement therapy, with the measured gabapentin clearance ∼80% of the measured urea clearance. 11 In this case report, we describe the successful management of gabapentin toxicity with continuous renal replacement therapy and calculate the clearance of gabapentin which will enable future treatment of gabapentin toxicity by CVVHD. We report a rare case with gabapentin overdose that caused severe rhabdomyolysis and acute tubular necrosis which required renal replacement therapy. A better awareness of its adverse effect and a close follow-up of laboratory tests are recommended. in predicting drug handling in both renal impairment and renal replacement therapy. • as those interactions which are potentially serious, Metabolism: Very few drugs are 100% excreted via either the liver or the kidneys. Many are metabolised by the liver to either active or inactive metabolites, and some of these may be excreted via the kidneys. of gabapentin clearance by continuous renal replacement therapy (CRRT) and provide, for the first time, clearance estimates of gabapentin. This information will be useful in prescribing adequate renal replacement therapy in patients with gabapentin toxicity. Acute kidney injury (AKI) requiring continuous renal replacement therapy (CRRT) is a common complication in critical illness and has a significant impact on pharmacokinetic factors determining drug exposure, including absorption, distribution, transport, metabolism, and clearance. In this review, we Gabapentin is a relatively safe medication, but in certain clinical scenarios, particularly in impaired renal functions, can lead to severe complications. Moreover, it per se can rarely lead to rhabdomyolysis and AKI. Isla A, Maynar J, Sanchez-Izquierdo JA, et al. Meropenem and continuous renal replacement therapy: in vitro permeability of 2 continuous renal replacement therapy membranes and influence of patient renal function on the pharmacokinetics in critically ill patients. J Clin Pharmacol 2005;45:1294–1304. We report a rare case with gabapentin overdose that caused severe rhabdomyolysis and acute tubular necrosis which required renal replacement therapy. A better aware-ness of its adverse effect and a close follow‐up of laboratory tests are recommended. Prescribers should also be aware of high‐risk population and monitor for signs of abuse Continuous renal replacement therapy (CRRT) is used in approximately 5% to 10% of patients with AKI, and affects medication clearance. 4 The optimal intensity of renal replacement therapy in critical illness is debated, but a prospective, multicenter, randomized trial demonstrated no significant impact of higher-intensity renal replacement We report a rare case with gabapentin overdose that caused severe rhabdomyolysis and acute tubular necrosis which required renal replacement therapy. A better awareness of its adverse effect and a close follow-up of laboratory tests are recommended. Prescribers should also be aware of high-risk popu Acute kidney injury (AKI) requiring continuous renal replacement therapy (CRRT) is a common complication in critical illness and has a significant impact on pharmacokinetic factors determining drug exposure, including absorption, distribution, transport, metabolism, and clearance. INTRODUCTION. Kidney replacement therapy (KRT) is commonly required in patients with severe acute kidney injury (AKI). Acute KRTs include standard intermittent hemodialysis, peritoneal dialysis, continuous kidney replacement therapies (CKRTs), and hybrid therapies such as prolonged intermittent kidney replacement therapies (PIKRT), still most widely known under the term prolonged intermittent In this case report, we describe the successful management of gabapentin toxicity with continuous renal replacement therapy and calculate the clearance of gabapentin which will enable future treatment of gabapentin toxicity by CVVHD. This case shows that gabapentin can induce rhabdomyolysis in healthy patients and that clinicians must consider the possible association between gabapentin and rhabdomyolysis. Keywords: Gabapentin; renal replacement therapy; rhabdomyolysis. Acute kidney injury (AKI) develops in up to 25% of patients in the ICU; 6% of ICU patients who develop AKI require renal replacement therapy (RRT) [4]. Approximately half of these patients receive continuous RRT (CRRT), largely owing to improved hemodynamic stability with this modality [5].
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |