Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 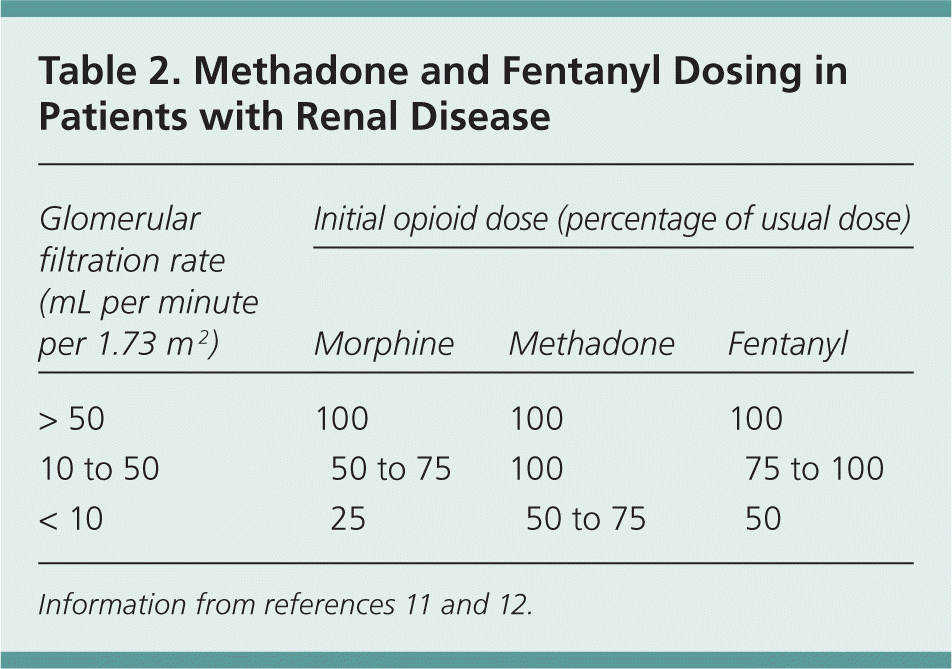 |
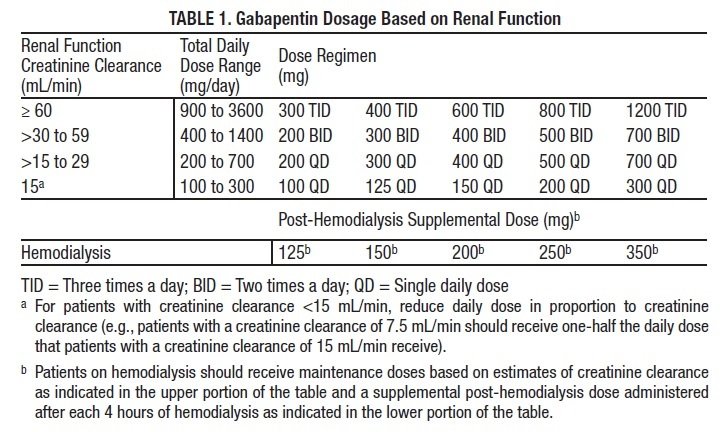
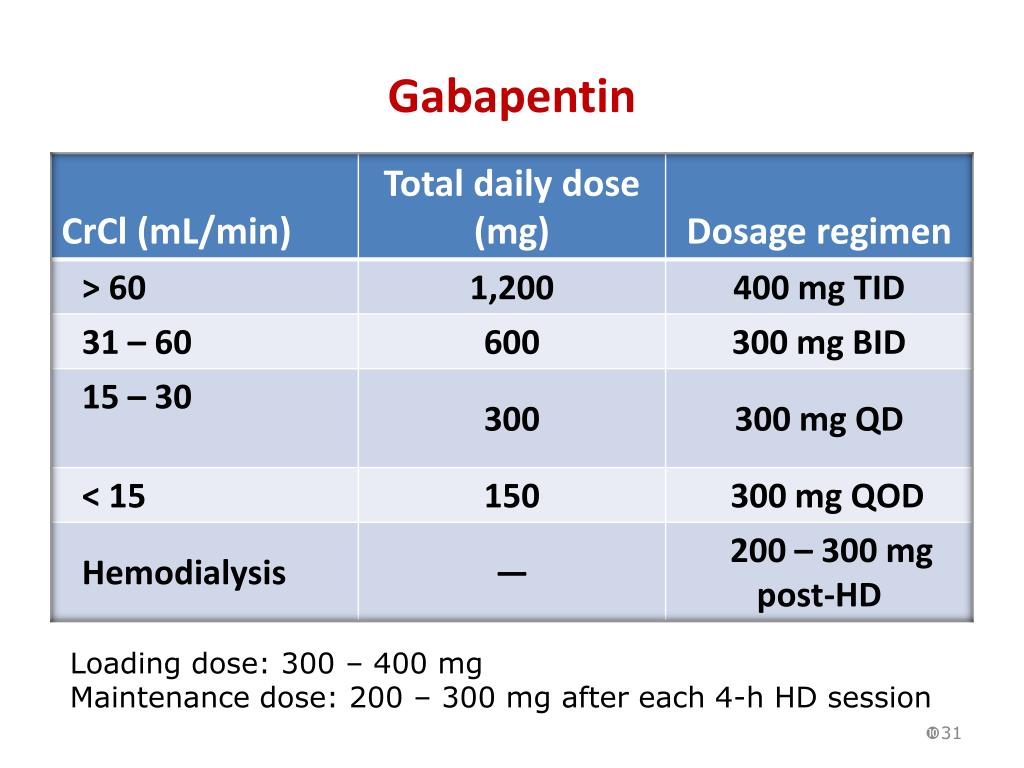
This study demonstrates that gabapentin dosage for patients with chronic kidney disease has been insufficiently adjusted and that the risk of gabapentin toxicity has been underrecognized. Gabapentin (C 9 H 17 NO 2 ) is a water-soluble 1-(aminomethyl)-cyclohexaneacetic acid and a structural analogue of the inhibitory neurotransmitter γ Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. Background: Gabapentinoids (GPs) are frequently prescribed in individuals with chronic kidney disease (CKD); however, their exclusive renal elimination warrants dose adjustments to decrease risk of toxicity. This study evaluated GP prescribing patterns and whether excessive dosing was associated with increased incidence of gabapentinoid-related Myoclonic activity may occur as a complication of gabapentin toxicity, especially with acute kidney injury or end-stage renal disease. We report 2 cases of myoclonic activity associated with gabapentin toxicity in the setting of renal disease which resolved with discontinuation of gabapentin and treatment with hemodialysis and peritoneal dialysis. Gabapentin toxicity should be considered one of the differential diagnoses of altered consciousness in patients with compromised renal function, even after a single dose. We report a 57-year-old woman with diabetes mellitus and uraemia on regular haemodialysis who developed severe dizziness and lethargy after a single recommended dose of Gabapentin is almost exclusively cleared by the kidney and thus presents challenges in patients with kidney failure. Gabapentin is known to be effectively cleared by hemodialysis, but the efficiency of clearance by peritoneal dialysis (PD) has not been previously described. We report a case of gabapentin toxicity in a patient Myoclonus is a well-reported complication of gabapentin toxicity especially in patients with renal impairment. As gabapentin is solely excreted by the kidneys, renal dose adjustment is recommended in the literature. Gabapentinoids are opioid substitutes whose elimination by the kidneys is reduced as kidney function declines. To inform their safe prescribing in older adults with chronic kidney disease (CKD), we examined the 30-day risk of serious adverse events according to the prescribed starting dose. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Rationale & Objective: Gabapentinoids are opioid substitutes whose elimination by the kid-neys is reduced as kidney function declines. To inform their safe prescribing in older adults with chronic kidney disease (CKD), we examined the 30-day risk of serious adverse events according to the prescribed starting dose. patients with decreased kidney function (quality of the evidence grade as A and B, respectively). Limited clinical data were available (24 patients with gabapentin toxicity and 7 with pregabalin toxicity received ECTR). Severe toxicity, mortality, and sequelae were rare in cases receiving ECTR and in historical controls receiving standard care Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its well recieved pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. People with chronic kidney disease who take gabapentin should be very aware of Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. The range of Gabapentin toxicity across the spectrum of renal insufficiency is underrecognized. In this case report, the patient with AKI only was confused and the patient with ESRD had myoclonus and seizure in addition to confusion. There seems to be a graded increase in toxicity with the corresponding deterioration of renal function. Discussion: Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Renal adjustments for the gabapentinoids are prodigiously recommended in the literature. However, current guidance is based on pharmacokinetic and toxicity studies, but studies confirming efficacy of these dosing strategies are lacking. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. toxic manifestations. Gabapentin toxicity was suspected initially in only 41.5% of symptomatic cases. CONCLUSION: Gabapentin toxicity in patients with chronic kidney disease is underrecognized. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, The short answer is: yes, gabapentin can be problematic for individuals with kidney failure and chronic kidney disease (CKD). While gabapentin is often prescribed for pain management, particularly nerve pain, and sometimes for seizures, its primary elimination pathway is through the kidneys. Exposure: Higher-dose gabapentinoids (gabapentin >300 mg/d or pregabalin >75 mg/d) versus lower-dose gabapentinoids (gabapentin ≤300 mg/d or pregabalin ≤75 mg/d). Outcomes: The primary composite outcome was the 30-day risk of a hospital visit with encephalopathy, a fall, or a fracture or a hospitalization with respiratory depression.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |