Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | %20for%20Restless%20Legs%20Syndrome.png?md=1) |
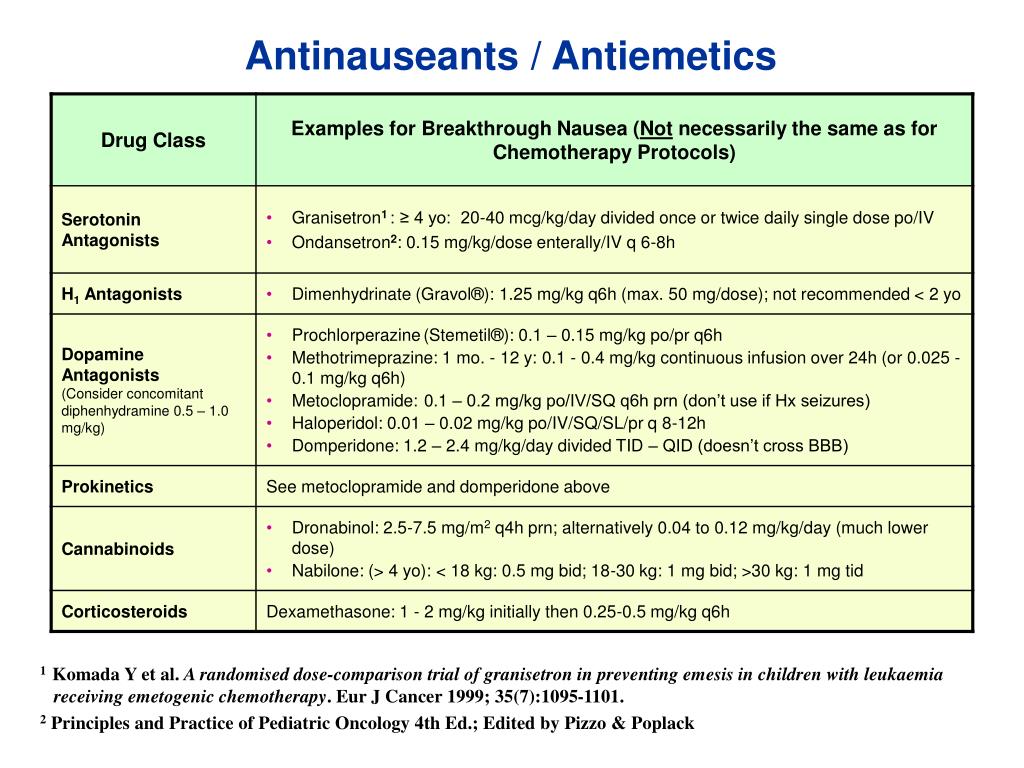 |  |
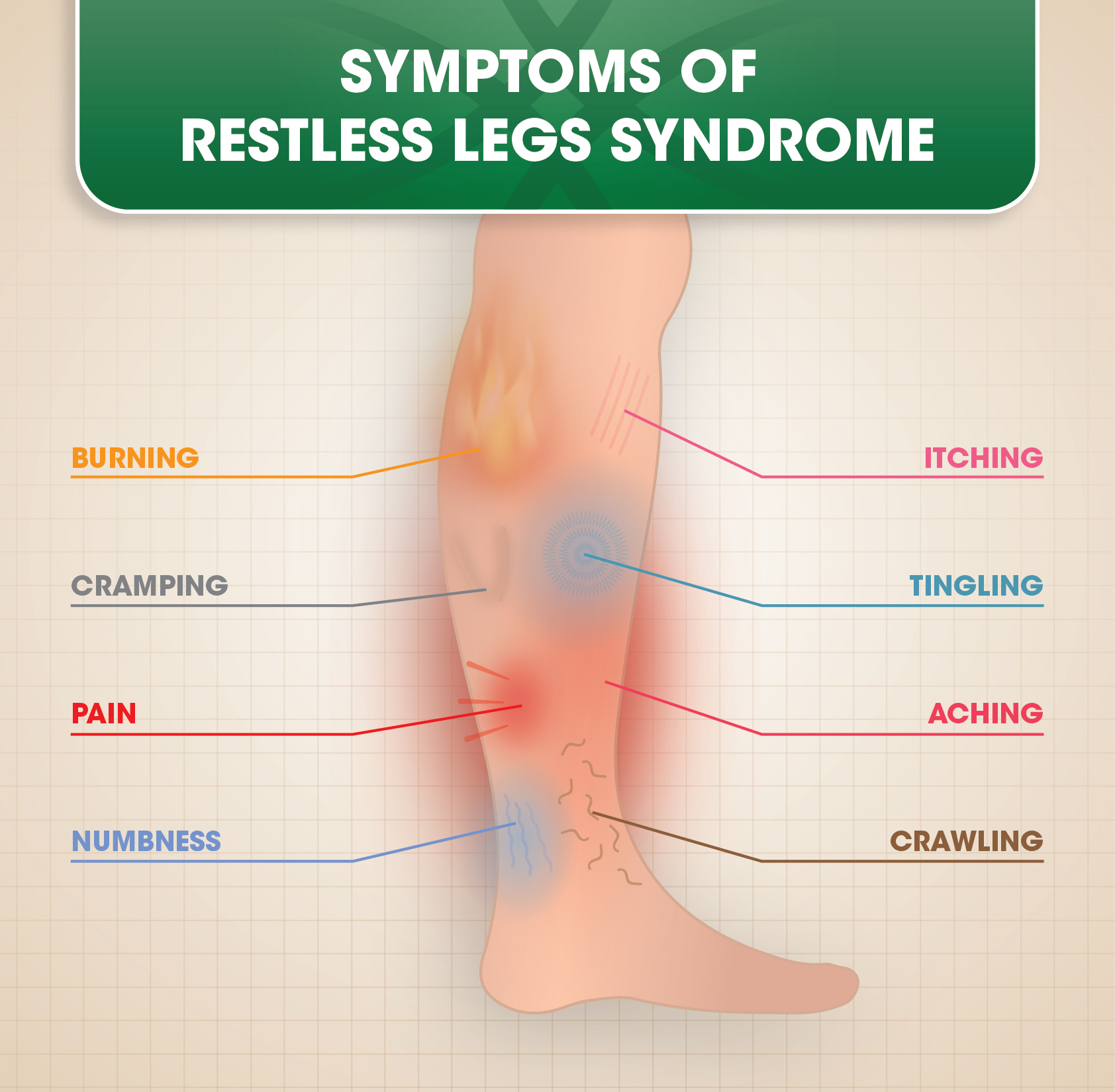
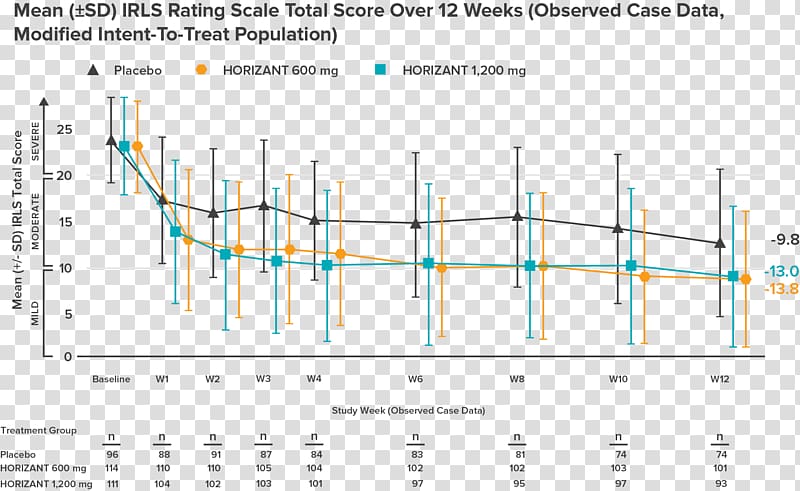
Gabapentin enacarbil is an extended-release prodrug of gabapentin that is approved in the USA (Horizant (®)) and Japan (Regnite (®)) for the treatment of moderate to severe primary restless legs syndrome (RLS) in adults [featured indication]. Gabapentin, primarily used for seizures and nerve pain, is also employed for Restless Legs Syndrome (RLS). It affects nerve signalling rather than muscles. Gabapentin’s effectiveness for RLS may take weeks, with dosage ranging from 300 mg to 3,600 mg daily. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (revised 2017). Mov Disord. 2018;33(7):1077–1091. Crossref Google Scholar; 27. Allen RP, Bharmal M, Calloway M. Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Objective: To assess the effects of gabapentin on sensory and motor symptoms in patients with restless legs syndrome (RLS). Methods: Patients with RLS (22 idiopathic, 2 secondary to iron deficiency) were randomized and treated for 6 weeks with either gabapentin or placebo. In contrast, new evidence supporting three alpha-2-delta ligand calcium channel blockers — gabapentin enacarbil, gabapentin, and pregabalin — led the task force to support them as strong recommendations for RLS treatment. These medications are not associated with the augmentation of RLS symptoms observed with the dopaminergic agents. Gabapentin is an effective and safe treatment for restless legs syndrome, with low levels in infant serum and long-term tolerability. A. Gabapentin enacarbil (Horizant) has been approved by the FDA for the treatment of restless legs syndrome (RLS) and postherpetic neuralgia (the pain that can linger after a bout of shingles). It is different from plain gabapentin ( Neurontin or Gralise ). Objective: To assess the effects of gabapentin on sensory and motor symptoms in patients with restless legs syndrome (RLS). Methods: Patients with RLS (22 idiopathic, 2 secondary to iron deficiency) were randomized and treated for 6 weeks with either gabapentin or placebo. Gabapentin enacarbil (marketed as Horizant) carries an FDA indication for the treatment of restless legs syndrome at a dose of 600 mg in the early evening, although FDA-approved doses of 1200 mg are permitted for other indications and used in some of the RLS clinical trials. This article explains what gabapentin is, its approved and off-label uses, and how the drug works to treat restless legs syndrome and other medical conditions. It also describes the possible side effects and risks and lists other drugs and treatments that may help ease RLS symptoms. Introduction. Restless legs syndrome (RLS) or Willis-Ekbom disease is a sleep-related movement disorder characterized by an irresistible urge to move, which usually involves the legs, although other parts of the body could also be involved. 1, 2 The four essential criteria used for the diagnosis of RLS are an urge to move the legs with or without abnormal sensations, worsening of symptoms at Restless legs syndrome (RLS) is a common disorder. The population prevalence is 1.5% to 2.7% in a subgroup of patients having more severe RLS with symptoms occurring 2 or more times a week and causing at least moderate distress. It is important for primary care physicians to be familiar with the disorder and its management. Much has changed in the management of RLS since our previous revised Restless legs syndrome (RLS) is characterized by an urge to move the legs, usually in association with limb discomfort. 1 The symptoms occur at rest, are relieved by movement, and are worse in the evening and at night. 1. Doan TT, Koo BB, Ogilvie RP, et al: Restless legs syndrome and periodic limb movements during sleep in the Multi-Ethnic Study of Atherosclerosis. Sleep 41(8):zsy106, 2018. doi: 10.1093/sleep/zsy106 A. Gabapentin enacarbil (Horizant) has been approved by the FDA for the treatment of restless legs syndrome (RLS) and postherpetic neuralgia (the pain that can linger after a bout of shingles). It is different from plain gabapentin (Neurontin or Gralise). The use of gabapentin for restless legs syndrome (RLS) is off-label. Initial dose of 300 mg if the person is under 65 years old and 100 mg if the person is over 65 years old. Maximum recommended dose for RLS is 2700 mg. CKS did not identify any specific guidance on dose titration for use in RLS. Gabapentin has been shown to improve RLS in a small number of clinical studies, but is limited by its short half-life and variable bioavailability. Gabapentin enacarbil is a novel prodrug of gabapentin designed to overcome these pharmacokinetic limitations. The FDA approved gabapentin enacarbil in 2011 as the first non-dopaminergic agent for the treatment of restless legs syndrome (RLS) symptoms. Although gabapentin enacarbil is a pro-drug of gabapentin, its pharmacokinetics differ.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | %20for%20Restless%20Legs%20Syndrome.png?md=1) |
 |  |