Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
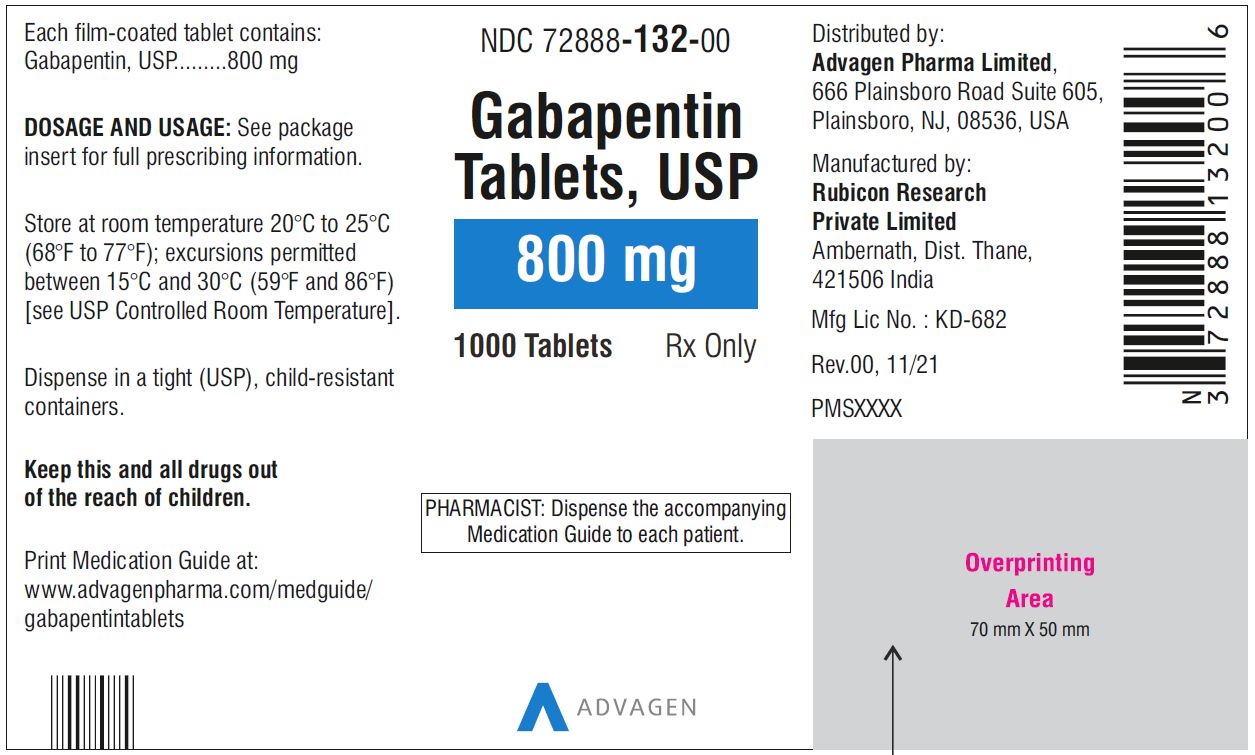
GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). gabapentin Antiepileptic Agent Sanis Health Date of Revision: August 22, 2018 IMPORTANT: PLEASE READ. This leaflet is part III of a three-part "Product Monograph" published when GABAPENTIN was approved for. sale in Canada and is designed specifically for Consumers. This leaflet is a summary and will not tell you. everything about GABAPENTIN. Gabapentin belongs to the class of medications called anti-epileptics. It is used in combination with other seizure control medications to manage and prevent seizures associated with epilepsy. Gabapentin does not cure epilepsy and only works to control seizures as long as the medication is taken. SANIS HEALTH INC; Details for: GABAPENTIN Drug and Health Product Portal The Product Monograph is a scientific document that describes the properties, claims GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. This leaflet is part III of a three-part “Product Monograph” published when GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. This leaflet is a summary and will not tell you pms-GABAPENTIN Product Monograph Page 3 of 29 Pr pms-GABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg and 400 mg Gabapentin Tablets, USP 600 mg and 800 mg PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Dosage Form / Strength All Nonmedicinal Ingredients Oral Capsules: 100 mg, 300 GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Geriatrics (> 65 years of age) women. Gabapentin should only be used during pregnancy if the potential benefit to the mother outweighs the potential risk to the fetus. Nursing Women: Gabapentin is excreted in human milk. Because the effect on the nursing infant is unknown, caution should be exercised when gabapentin is administered to a nursing mother. Gabapentin produced an increased incidence of acinar cell adenomas and carcinomas in the pancreas of male rats, but not female rats or in mice, in oncogenic studies with doses of 2000 mg/kg which resulted in plasma concentrations 14 times higher than those occurring in humans TEVA-GABAPENTIN (gabapentin) Product Monograph Page 7 of 32 administration was revealed in reproduction studies in mice at doses up to 62 times, and in rats and rabbits at doses up to 31 times the human dose of 2400 mg/day. GABAPENTIN Product Monograph Page 3 of 32 PrGABAPENTIN Gabapentin Capsules, House Standard 100 mg, 300 mg and 400 mg PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Dosage Form / Strength All Non-medicinal Ingredients Oral Capsules: 100 mg, 300 mg, and 400 mg Corn Starch, Lactose Anhydrous, and Talc. The Product Monograph is a scientific document that describes the properties, claims, indications and conditions of use of the product and contains any other information that may be required for optimal, safe and effective use. For a complete listing, see 6 DOSAGE FORMS, STRENGTHS, COMPOSITION AND PACKAGING. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation. 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance >60 mL/min) to 52 hours (creatinine clearance <30 mL/min) and This leaflet is part III of a three-part "Product Monograph" published when GABAPENTIN was approved for sale in Canada and is designed specifically for Consumers. Product Monograph GABAPENTIN Page 1 of 31 PRODUCT MONOGRAPH PrGABAPENTIN Gabapentin Capsules (Manufacturer’s Standard) 100 mg, 300 mg and 400 mg Antiepileptic Agent Sivem Pharmaceuticals ULC 4705 Dobrin Street Saint-Laurent, Quebec H4R 2P7 www.sivem.ca Date of Revision: August 27, 2018 Submission Control No.: 219015 Gabapentin de Sanis: La gabapentine appartient à la classe des médicaments appelés antiépileptiques. Elle s'utilise en association avec d'autres médicaments de même nature pour le traitement et la prévention des crises d'épilepsie. La gabapentine ne guérit pas l'épilepsie; elle maîtrise les crises seulement pendant les périodes où le médicament est pris. Le médicament agit en pms- GABAPENTIN (gabapentine) n'est pas jugé efficace contre les crises à type d'absence et doit donc être employé avec prudence chez les patients dont l'épilepsie est mixte et qui ont des absences. Arrêt du traitement par pms -GABAPENTIN . Comme pour les autres anticonvulsivants, il n'est pas recommandé de cesser brusquement Concomitant use of CNS depressants with gabapentin is also a contributing factor. Concomitant Use With Opioids Concomitant use of opioids with Gabapentin potentiates the risk of respiratory depression, profound sedation, syncope, and death. Gabapentin concentrations may also increase in patients receiving concomitant opioid (See DRUG INTERACTIONS).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |