Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
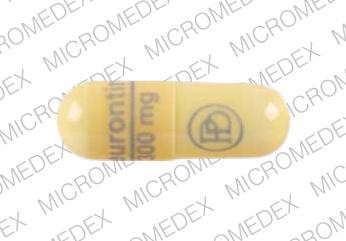 |  |
Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Schedule II. Schedule II drugs, substances, or chemicals are defined as drugs with a high potential for abuse, with use potentially leading to severe psychological or physical dependence. These drugs are also considered dangerous. 95% of patients receive gabapentin for off-label indications.2 The off-label indications from this review include pain, bipo-lar affectivedisorder, restless leg syndrome, multiple sclerosis, anxiety, and other behavioral disorders.2 Gabapentin mayalso be used off label for alcohol use disorder, alcohol withdrawal, Step #1: Patient requires an Emergency Schedule II prescription. Step #2: Nurse Contacts the Practitioner. Emergency Oral Schedule II Prescription Process. U.S. Drug Enforcement Administration Diversion Control Division pursuant to the texas controlled substances act, health and safety code, chapter 481, these schedules supercede previous schedules and contain the most current version of the Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Schedule 2 (II) Drugs. The drug has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence. While all other Schedule 3 CDs, including tramadol, pentazocine, the barbiturates, gabapentin, and pregabalin, as well as Schedule 2 drug quinalbarbitone are not subject to the same Safe Custody Regulations, it is an RCVS requirement that they are securely locked away. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. 37-2707. Schedule II. (a) Schedule II shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. No Schedule I substances; Up to a 3-day supply of a Schedule II narcotic; non-narcotic Schedule II’s may be prescribed just like a physician; No other limitations other than the Opioid Reduction Act (Can be found in the Pharmacy Law Book) Lost or Stolen Controlled Substances. As required by § 15-2-9.3.1: 1 . C. ONTROLLED SUBSTANCES – QUICK REFERENCE FOR SCHEDULE . Wisconsin Department of Health Services / Division of Quality Assurance . P-01807 (06/2023) A gabapentin taper chart can provide structure, helping you gradually reduce your dose while minimizing the discomfort that can come with stopping too quickly. It’s not about rushing—it’s about finding a steady, safe way forward that works for you. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. Note differences in KY schedule and federal schedule: pentazocine (Schedule III in KY, Schedule IV federally) nalbuphine (Schedule IV in KY, not scheduled federally) Day 2: 300 mg orally 2 times day Day 3: 300 mg orally 3 times a day. Titrate dose as needed for pain relief; Maintenance dose: 900 to 1800 mg/day orally in 3 divided doses Maximum dose: 1800 mg per day Extended-release: Gralise (gabapentin) 24-hour extended-release tablets: Initial dose: Day 1: 300 mg orally with the evening meal • Schedule V drugs have an even lower potential for abuse, but may include a limited quantity of narcotics. Examples include some cough medicines (Robitussin AC) and Lyrica. WHAT DRUGS DO PDMPs MONITOR? PDMPs track Schedule II, III and IV drugs in every state, and Schedule V drugs in 33 states and D.C., including Pennsylvania. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |