Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 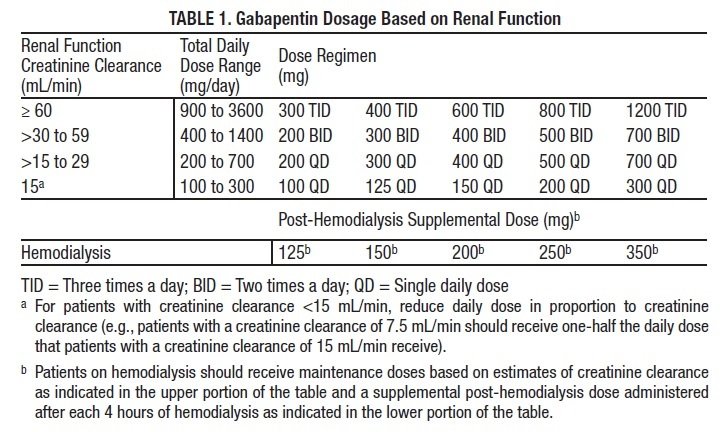 |
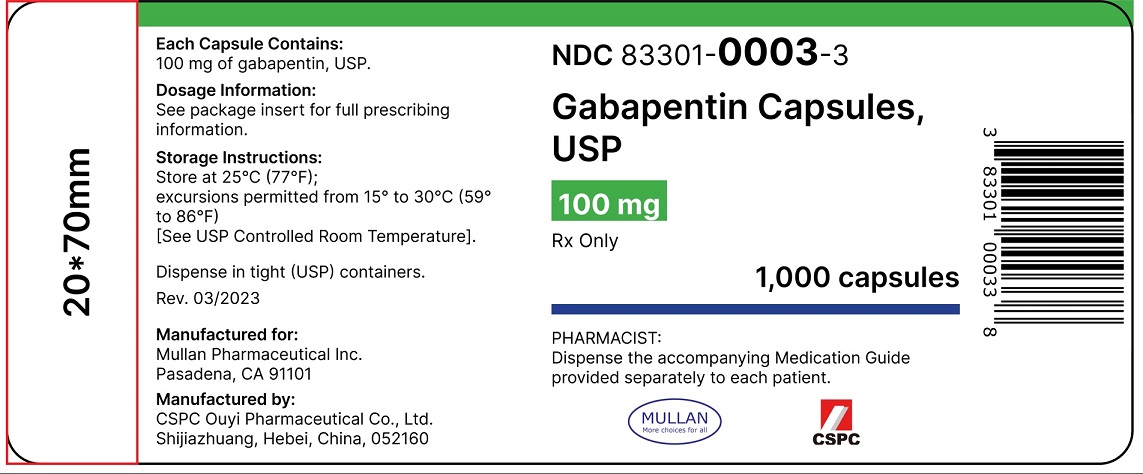
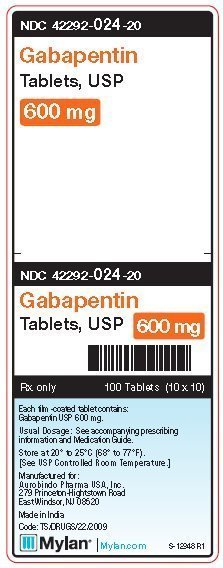
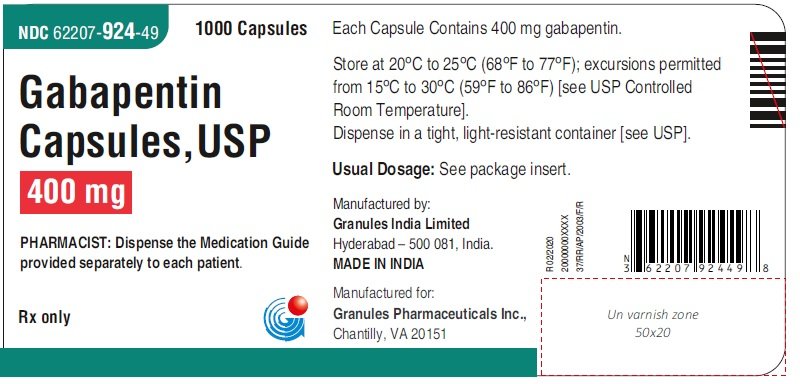
Controlled Drugs and Substances Act (Police Enforcement) Regulations (SOR/97-234) Narcotic Control Regulations (C.R.C., c. 1041) New Classes of Practitioners Regulations (SOR/2012-230) Precursor Control Regulations (SOR/2002-359) Controlled Drugs and Substances Act. S.C. 1996, c. 19. Assented to 1996-06-20. An Act respecting the control of certain drugs, their precursors and other substances and to amend certain other Acts and repeal the Narcotic Control Act in consequence thereof Offences under s. 4(4) CDSA [possession of controlled substances – Schedule II (cannaboids)] are absolute jurisdiction offences under s. 553(a) and so does not have a defence election of court. Tell your doctor immediately if you experience increased side effects such as drowsiness or slowed breathing while taking GABAPENTIN with an opioid or other sedatives and tranquilizers. The dose of these drugs or GABAPENTIN may need to be adjusted. Avoid alcoholic drinks while taking GABAPENTIN. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. The Controlled Substances Act categorizes certain medications into 5 different schedules based on misuse potential. Schedule I medications have the highest misuse potential, and Schedule V medications have the lowest misuse potential.[1] Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Pursuant to subsection 56(1) of the Controlled Drugs and Substances Act (CDSA), and subject to the terms and conditions herein, practitioners and pharmacists, authorized within their scope of practice, are hereby exempted from the following provisions of the CDSA and its regulations when prescribing, All legislative documents related to controlled substances are contained in the Controlled Drugs and Substances Act, the Narcotic Control Regulations, Parts G and J of the Food and Drug Regulations, and the Benzodiazepines and Other Targeted Substances Regulations. Canadian legislation has been developed in compliance with the UN Single HTML Full Document: Controlled Drugs and Substances Act (Accessibility Buttons available) | SCHEDULE V (Sections 2, 5 to 7.1, 10, 55 and 60.1) Column 1 Column 2; XML Full Document: Controlled Drugs and Substances Act [388 KB] | PDF Full Document: Controlled Drugs and Substances Act [710 KB] Act current to 2025-03-03 and last amended on 2024-09-03. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Gabapentin is chemically known as 2-[1-(aminomethyl) cyclohexaneacetic acid]. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The CDSA forbids the following activities for controlled substances and precursors, unless authorized by regulations or exempted under the CDSA: sale; export; import; possession; production; The regulations and exemptions authorize lawful activities for medical, scientific and industrial purposes. Some of the regulations made under the CDSA are: As the drug schedule changes-- Schedule II, Schedule III, etc., so does the abuse potential-- Schedule V drugs represents the least potential for abuse. A Listing of drugs and their schedule are located at Controlled Substance Act (CSA) Scheduling or CSA Scheduling by Alphabetical Order. Examples of Schedule V substances include: cough preparations containing not more than 200 milligrams of codeine per 100 milliliters, atropine / diphenoxylate (Lomotil), pregabalin (Lyrica) and ezogabine. From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Sentencing. Marginal note: Purpose of sentencing 10 (1) Without restricting the generality of the Criminal Code, the fundamental purpose of any sentence for an offence under this Part is to contribute to the respect for the law and the maintenance of a just, peaceful and safe society while encouraging rehabilitation, and treatment in appropriate circumstances, of offenders and acknowledging SCHEDULE VI (Sections 2, 6, 55 and 60) PART 1 Class A Precursors [1] 1 Acetic anhydride. 2 N-Acetylanthranilic acid (2-acetamidobenzoic acid) and its salts 3 Anthranilic acid (2-aminobenzoic acid) and its salts 4 Ephedrine (erythro-2-(methylamino)-1-phenylpropan-1-ol), its salts and any plant containing ephedrine or any of its salts teva-gabapentin (gabapentin) page 1 of 41 product monograph including patient medication information
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |