Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
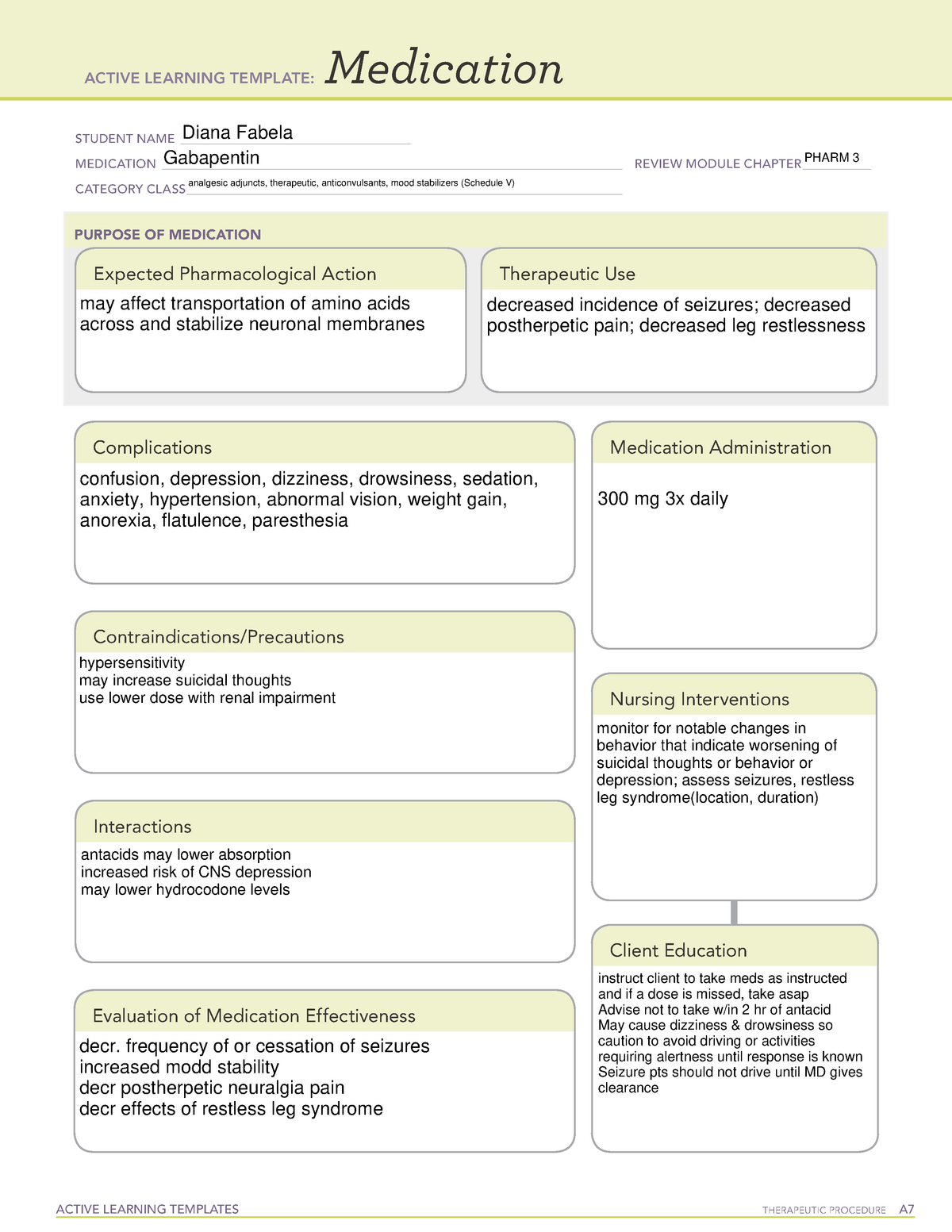
Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. AMENDMENT S that aected UCA 58-37-4 Schedules of con trolled substances and other . licensure board regulations will apply t o Gabapentin. Please review all con trolled substance security, storage, record keeping, inventor y, prescribing, and dispensing requirements. This document is not intended to be an all-inclusive overview. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk of overdose Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Public Citizen, a non-profit advocacy group, has stated that recent studies showed that gabapentin increases the risk of opioid overdose death. With the opioid epidemic at an all-time high, the federal government is keeping a close eye on gabapentin and other substances that could potentially be abused. Symptoms of Gabapentin Addiction because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin [It’s] because gabapentin originally was approved for partial onset seizures back in 1993. Following that, a very similar drug, small molecule drug pregabalin, brand name Lyrica, was approved for some similar indications in 2005. Pregabalin was scheduled and is still on schedule V. Gabapentin is not, going back to 1993. So why now? Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. FDA announced a new mandate that labels of gabapentin and pregabalin contain a warning about respiratory depression . ii Pregabalin is controlled in Schedule V of the Federal Controlled Substances Act . Although not controlled federally, some States also list gabapentin as a Schedule V controlled (a) [(1)] Any compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth in this subsection, which also contains one or more nonnarcotic active medicinal ingredients in sufficient proportion to confer upon the compound, mixture, or preparation, valuable medicinal Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Background Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear. Objective To determine the effect of state-imposed Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Our data support that this policy is effective at lowering rates of gabapentin prescribing in the Medicare Part D enrollee population. To support the national imperative to reduce gabapentin prescribing, the FDA may consider changing the federal gabapentin schedule classification to Schedule V. Limitations (a) Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Each drug or substance has been assigned the DEA Controlled Substances Code Number set forth opposite it. (b) Opiates. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |