Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
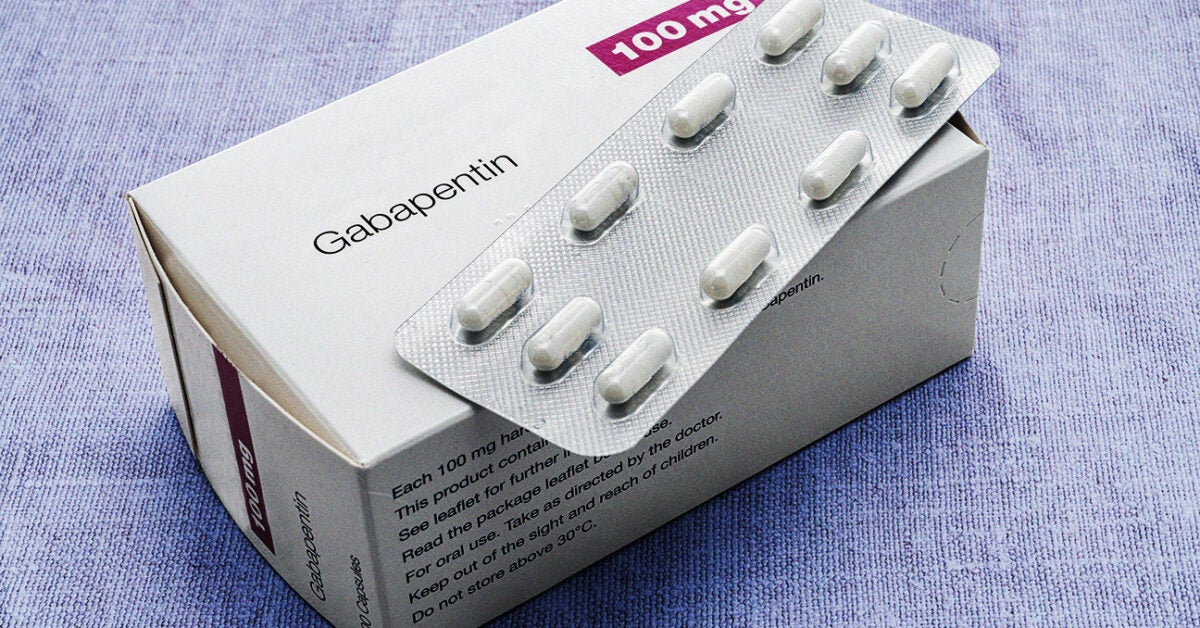
The General Assembly's Illinois Administrative Code database includes only those rulemakings that have been permanently adopted. This menu will point out the Sections on which an emergency rule (valid for a maximum of 150 days, usually until replaced by a permanent rulemaking) exists. (a) The controlled substances listed in this Section are included in Schedule I. (b) Unless specifically excepted or unless listed in another schedule, any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, whenever the existence of such isomers, esters, ethers and salts is (720 ILCS 570/204) (from Ch. 56 1/2, par. 1204) Sec. 204. (a) The controlled substances listed in this Section are included in Schedule I. (b) Unless specifically excepted or unless listed in another schedule, any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, whenever the existence of such isomers, esters, ethers and salts All Schedule II-V prescriptions (12-month history of all CII-V prescriptions dispensed in an Illinois retail pharmacy) Additional drugs of interest prescriptions that are mandated to be reported (12-month history of: Naltrexone, Naloxone, Butalbital/Acetaminophen/Caffeine, Muscle relaxants, Gabapentin) Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug Under the Illinois Controlled Substances Act narcotic and non-narcotic schedules are combined and the 'N' designation is not needed. The lack of 'N' designation does not prevent or affect the ability of Illinois licensed practitioners to prescribe, dispense or order narcotic drugs to the extent allowed by law. The DEA is aware of this change. Since the period of our study, three additional states, North Dakota, Michigan, and Virginia, have implemented Schedule V regulation, and two states, Illinois and Utah, added state-mandated PDMP requirements for gabapentin. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Gabapentin is structurally and pharmacologically related to pregabalin (Lyrica, Lyrica CR), which is a Schedule V drug and controlled federally in all states. Gabapentin is not a narcotic; however, according to the DEA, gabapentin has been increasingly documented as an illicit drug of abuse by police, in crime reports, and by U.S. poison In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. (a) The controlled substances listed in this Section are included in Schedule IV. (b) Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing limited quantities of any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, as set Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation States that have put gabapentin through prescription drug monitoring programs include Minnesota, Ohio, Illinois, Massachusetts, and Wyoming. While gabapentin is not considered a controlled substance in these states, putting the drug through PDMPs is a step towards minimizing the risks of abusing the medication. (720 ILCS 570/100) (from Ch. 56 1/2, par. 1100) Sec. 100. Legislative intent. It is the intent of the General Assembly, recognizing the rising incidence in the misuse of drugs and other dangerous substances and its resultant damage to the peace, health, and welfare of the citizens of Illinois, to provide a system of control over the distribution and use of controlled substances which will more The General Assembly's Illinois Administrative Code database includes only those rulemakings that have been permanently adopted. This menu will point out the Sections on which an emergency rule (valid for a maximum of 150 days, usually until replaced by a permanent rulemaking) exists. such as Gabapentin, as a reportable drug. Iowa has not scheduled Gabapenin as a controlled substance. Michigan: Michigan has scheduled Gabapentin as a Schedule V controlled substance. Michigan’s prescription monitoring program requires all Schedule II-V controlled substances to be monitored. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |