Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
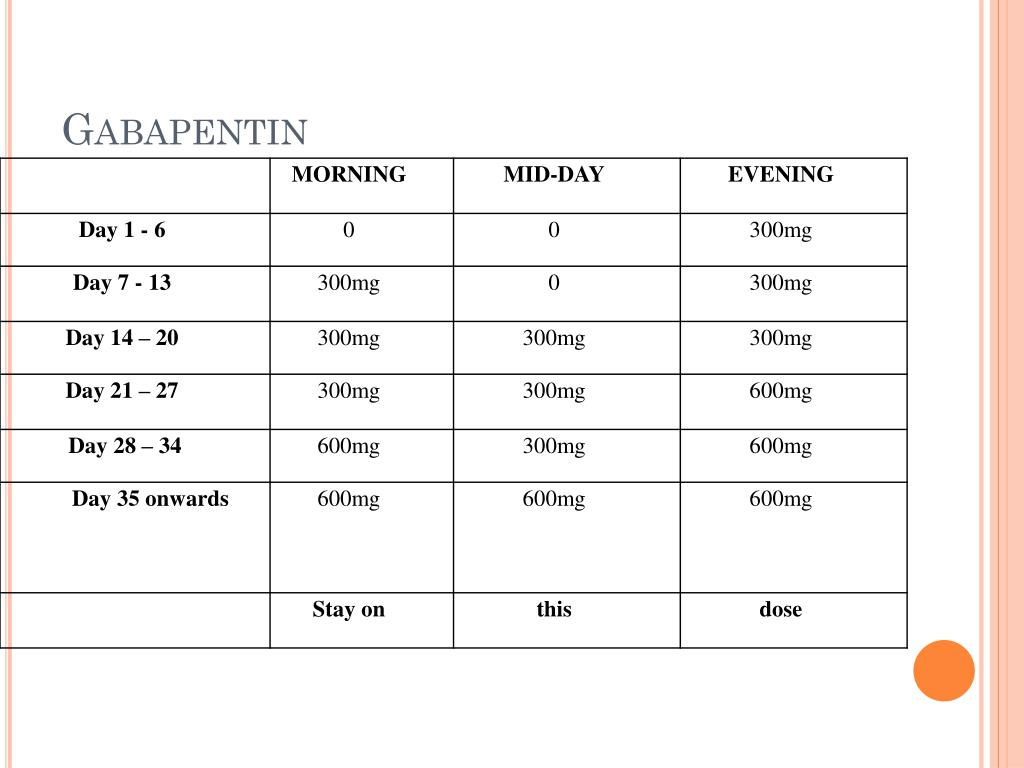
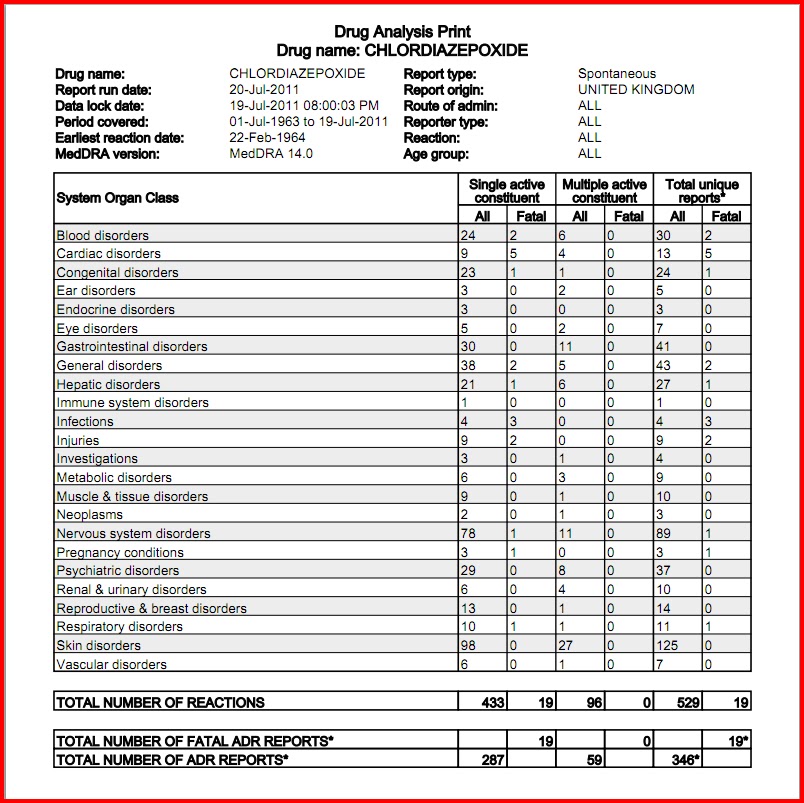
} v } o o ^ µ v W ] ] v P ' µ ] o ] v ( } WZE ] v < v µ l Ç ~ µ î X î ì î í d Z ] o ] ] v } o o r ] v o µ ] À X / ( Ç } µ Z À µ ] } v } } u u v o } v WW ñ ì î r ñ ò ð r ó õ ô ñ Background Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear. Objective To determine the effect of state-imposed Background: Owing to increasing concern over the potential for gabapentin misuse, gabapentin was reclassified as a schedule V controlled substance in Kentucky (July 2017). Objective: This study aimed to characterize gabapentin use among Kentucky residents in the first year after its scheduling. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- As of November 2020, seven states — Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia — had classified gabapentin as a schedule V drug, while another 12 states Kentucky will become the first state to change gabapentin to a Schedule V controlled substance, [] Tianeptine Now a Schedule I Controlled Substance in Kentucky Pharmacist CE Requirement 2023-2028: 1 Contact Hour on Opioid Epidemic or Opioid Use Disorder Required Each Year. USP Revisions 797 & 795: Public comment period now open for draft proposal of 201 KAR 2:076 compounding regulation. As a result, several states have rescheduled gabapentin to control its availability and limit its potential for harm. Healthcare providers must be vigilant when prescribing and dispensing the medication. This post aims to offer guidance to healthcare providers involved in prescribing and dispensing gabapentin. This study used Kentucky All Schedule Prescription Electronic Reporting data (2018). Gabapentin use was defined as having at least 1 dispensed gabapentin prescription, and high-dose gabapentin use was defined as an average daily dose of more than 3600 mg at the patient level. Effective July 1, 2017, all gabapentin products will be Schedule 5 controlled substances in Kentucky. All applicable provisions of KRS Chapter 218A, 902 KAR Chapter 55 and other licensure board regulations will apply to gabapentin. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Effective July 1, 2017, all gabapentin products will be Schedule 5 controlled substances in Kentucky. All applicable provisions of KRS Chapter 218A, 902 KAR Chapter 55 and other licensure board regulations will apply to gabapentin. What states consider gabapentin a controlled substance? As of July 2022, these states consider gabapentin a schedule V controlled substance: Alabama. Kentucky. Michigan. North Dakota. Tennessee. Virginia. West Virginia. Other states have mandated gabapentin reporting. Every time you fill a gabapentin prescription, it’s added to the PDMP As of July 1, 2017, gabapentin will be classified as a Schedule V, controlled substance (in KY). APRNs must now meet prescribing requirements for controlled substances in order to prescribe gabapentin. APRNs will no longer be able to prescribe gabapentin unless they have a DEA license and a CAPA-CS. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. gabapentin (Scheduled V in KY, not scheduled federally) barbital, methylphenobarbital and phenobarbital (Schedule III in KY, Schedule IV federally) --Be advised--KY does not exempt ANY butalbital product from the Control Substance Act (902 KAR 55:045) According to the federal government, gabapentin isn’t a controlled substance. But because its potential for misuse has become increasingly evident, some states (Kentucky being the first) have listed gabapentin as a Schedule V drug with the intent of restricting access and curtailing use without a prescription. But that’s not what happened. controlled substance and gabapentin as a Schedule V controlled substance. The Cabinet for Health and Family Services recognizes that nalbuphine and gabapentin have significant abuse potential, and inclusion on Kentucky’s controlled substances schedules will help reduce the risk to public health. Section 1. Schedule I Controlled Substances. In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death. 24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in This administrative regulation further differs from the federal regulation, 21 C.F.R. 1308.14-1308.15, because it designates nalbuphine as a Schedule IV controlled substance and gabapentin as a Schedule V controlled substance.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |