Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
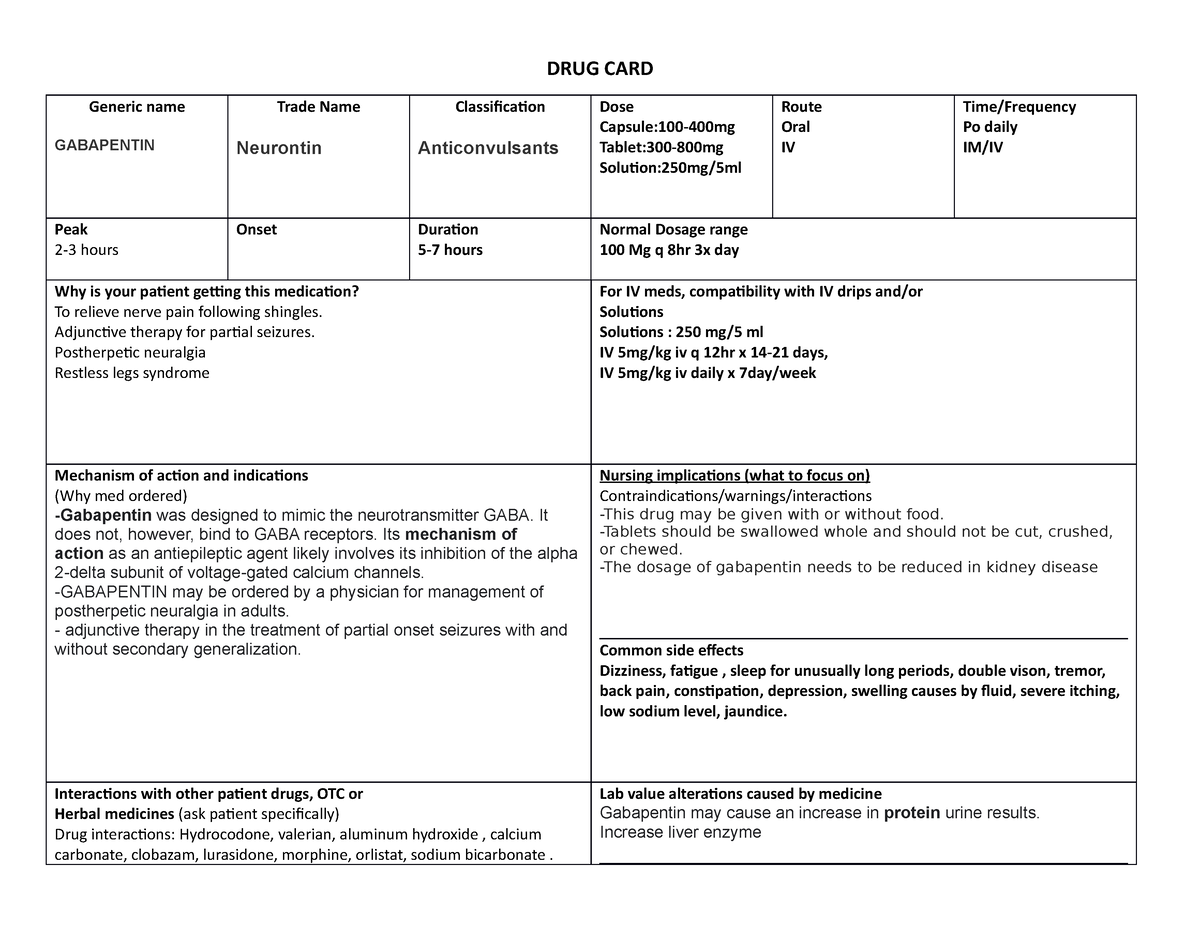
Gabapentin and MassPAT The Massachusetts Prescription Awareness Tool (MassPAT) is an online tool utilized by authorized pro-viders that supports safe prescribing and dispensing of Schedule II-V controlled substances (CS). It is part of the Massachusetts Prescription Monitoring Program and requires pharmacies to report prescribing and dispensing The MA PMP collects dispensing information on Massachusetts Schedule II - V controlled substances dispensed pursuant to a prescription. As of August 1, 2017 MA PMP required the reporting of Gabapentin (a Schedule VI medication) • The Department reviews and analyzes PDMP data to: Determine prescribing and dispensing trends; because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin Pharmacies are required to submit data to the PMP for all dispensations of Schedule II-V medications and Gabapentin, a Schedule VI medication designated by the Commissioner as a drug of concern. Please note, the Veterans Administration (VA) is limited by statute to report only dispensations of federally controlled substances in Schedules II-V. Some examples of drugs that would fit into Schedule VI are antibiotics and “maintenance meds,” such as penicillin, azithromycin, metoprolol, simvastatin, lisinopril, gabapentin, levothyroxine, metformin, etc. Pharmacies are required to submit dispensing information on federally controlled substances in Schedules II through V and gabapentin, a Massachusetts Schedule VI substance, within 24 hours or the next business day to the State of Massachusetts through the PMP Clearinghouse application provided by Bamboo Health, Inc. (Bamboo). In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). (a) Unless specifically excepted or unless listed in another schedule, any of the following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters and ethers, whenever the existence of such isomers, esters, ethers and salts is possible within the specific chemical designation: (1) Acetylmethadol (2) Allylprodine In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death. 24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. MA Vol. 2, No. 3 Page 1 Identification Requirements for CS . Prescriptions. A pharmacy that dispenses federally designated con-trolled substances (CS) and Schedule VI prescription monitoring program (PMP) drugs (eg, gabapentin) is re-quired to check that the photo identification (ID) matches the customer taking possession of the prescription 105 CMR 700 Implementation of the Controlled Substances Act (MGL c. 94C). 105 CMR 720 List of interchangeable drug products. 105 CMR 721 Standards for approved prescription forms (print and electronic) in Massachusetts Section 23: Written or electronic prescriptions; requirements and restrictions Section 23. (a) A written or electronic prescription for a controlled substance in Schedule II shall become invalid 30 days after the date of issuance. Massachusetts (MA) Reporting 24,25 : The reporting requirement shall apply to every pharmacy that dispenses gabapentin, and to any pharmacy in another state, commonwealth, district or territory that delivers such drug to a person in Massachusetts. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Talk to your healthcare provider about the risks of gabapentin before taking it. Dispensing Schedule II ePrescriptions – Endorsement M.G.L. c. 94C § 23(c) requires a pharmacist filling a Schedule II ePrescription to endorse his own signature on the face thereof. A pharmacist’s act of final verification of a Schedule II ePrescription shall fulfill this endorsement requirement. Partial Fill Online CME, Massachusetts Medical Society, “Identifying Drug Dependence” (FREE) Utilizing MassPAT. Massachusetts law requires that physicians must utilize the prescription-monitoring program prior to prescribing to a patient each time: a narcotic drug in Schedule II or III, and/or a benzodiazepine; OR any controlled substance in Scheduled IV or V which the Department has designated in The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- ÐÏ à¡± á> þÿ \ ^ þÿÿÿ
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |