Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
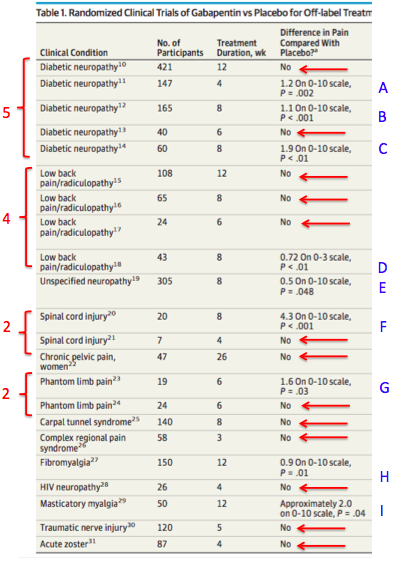
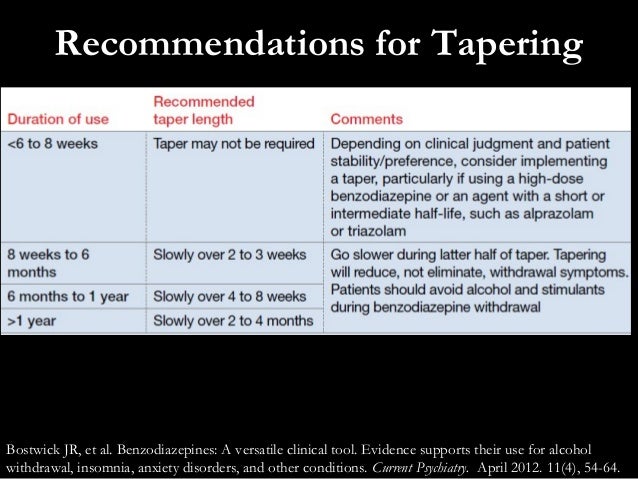
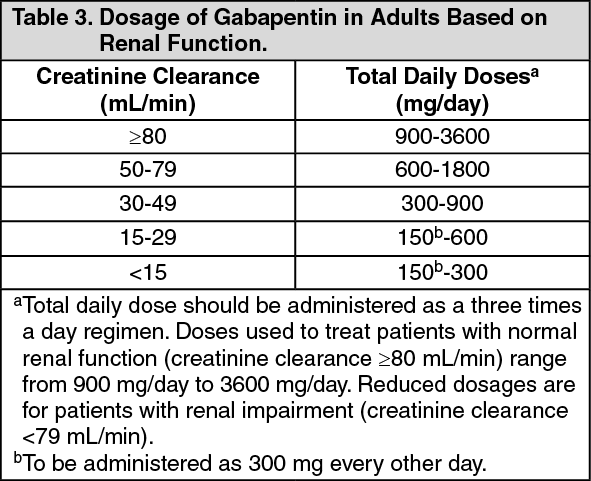
We would like to show you a description here but the site won’t allow us. We would like to show you a description here but the site won’t allow us. To help prevent prescription drug abuse and to protect the health and safety of our community, Pennsylvania's Prescription Drug Monitoring Program (PDMP) collects information on all dispensed controlled substance prescriptions. As of January 1, 2023 both physician assistants (PA) and APRNs will have the same prescriptive authority. Per §30-3E-3 and §30-7-15E, the new prescriptive authority for APRNs and PAs is as below: No Schedule I substances; Up to a 3-day supply of a Schedule II narcotic; non-narcotic Schedule II’s may be prescribed just like a physician of correlation between dose and serum concentration. Pa-tient age and weight have been found to affect gabapentin’s absorption. Not surprisingly, the estimated renal function also has been found to affect gabapentin’s elimination because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Background Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation into their own hands. The impact of these changes on gabapentin prescribing is unclear. Objective To determine the effect of state-imposed Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. In determining that a substance comes within this schedule, the Secretary will find: a high potential for abuse; no currently accepted medical use in the United States; and a lack of accepted safety for use under medical supervision. The following controlled substances are included in this schedule: (i) [Reserved]. (ii) Allylprodine. (4) Unless specifically excepted or unless listed in another schedule, a material, compound, mixture, or preparation which contains any quantity of the following substances including its salts, isomers, whether optical position or geometric, and salts of the isomers whenever the existence of the salts, isomers, and salts of isomers is possible within the specific chemical designation, Schedule Yes, Gabapentin is classified as a Schedule V controlled substance in Pennsylvania since 2020. It means that its use and distribution are regulated due to the potential for abuse and dependence. Patients must have a prescription from a licensed healthcare provider to legally possess and obtain it. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Pennsylvania's Department of Health issued a final order on June 3, 2023, temporarily scheduling xylazine as a Schedule III substance, which was not previously listed in any schedule of The Controlled Substance, Drug, Device and Cosmetic Act (35 P.S. §§ 780-101—780-144), subject to the limitations outlined in the final order. Although Gabapentin-related* Overdose Deaths† among Pennsylvania Residents, 2017-2020 Gabapentin-related Fatal and Non-fatal Drug Overdoses among Pennsylvania Residents, 2017-2020 Gabapentin was initially approved in 1993 by the U.S. Food and Drug Administration for the treatment of epilepsy; in 2004, it In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Gabapentin: Gabapentin is a drug used to treat seizures, pain, hot flashes and restless leg syndrome. 21 Although it does not present a high risk of fatal overdose, long-term effects have not been well researched. 22 The drug can avert opioid withdrawals and can stop the effects of • Gabapentin: Policymakers are increasingly interested in monitoring Gabapentin due to a recent uptick in Gabapentin prescriptions and its regular involvement in overdoses. As a drug that can curb opioid withdrawals and lessen the effects of medications used for addiction treatment, Gabapentin is widely misused. as gabapentin, its binding affinity to receptors in the brain and the potency of the medication is six times more than that of gabapentin.18,23 Furthermore, orally administered pregabalin is absorbed more rapidly in the body than gabapentin and remains present in the bloodstream for longer periods of time. The increased potency Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |