Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
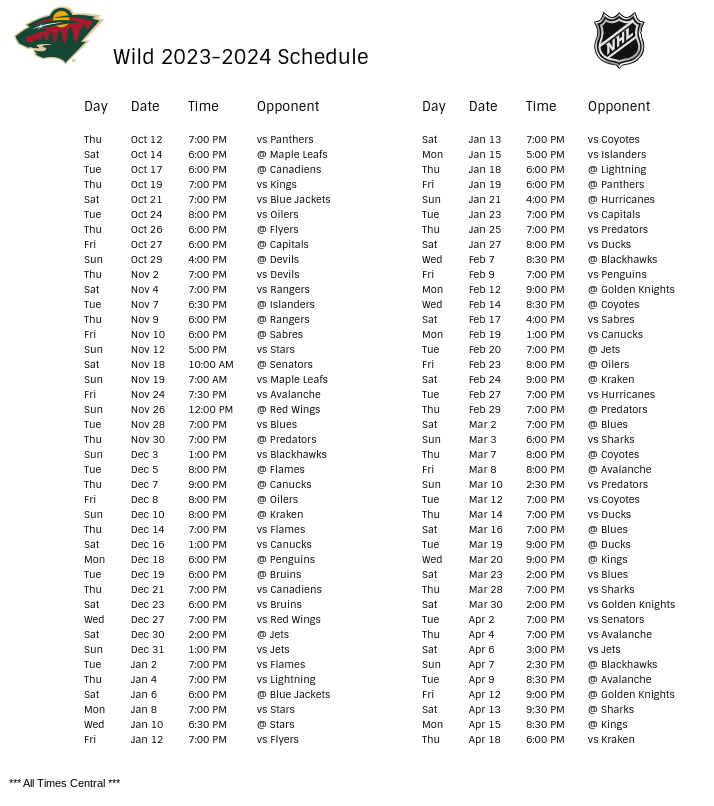 | 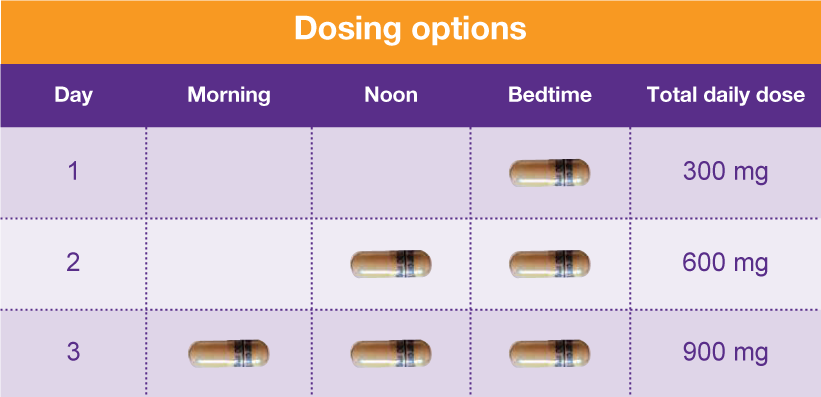 |
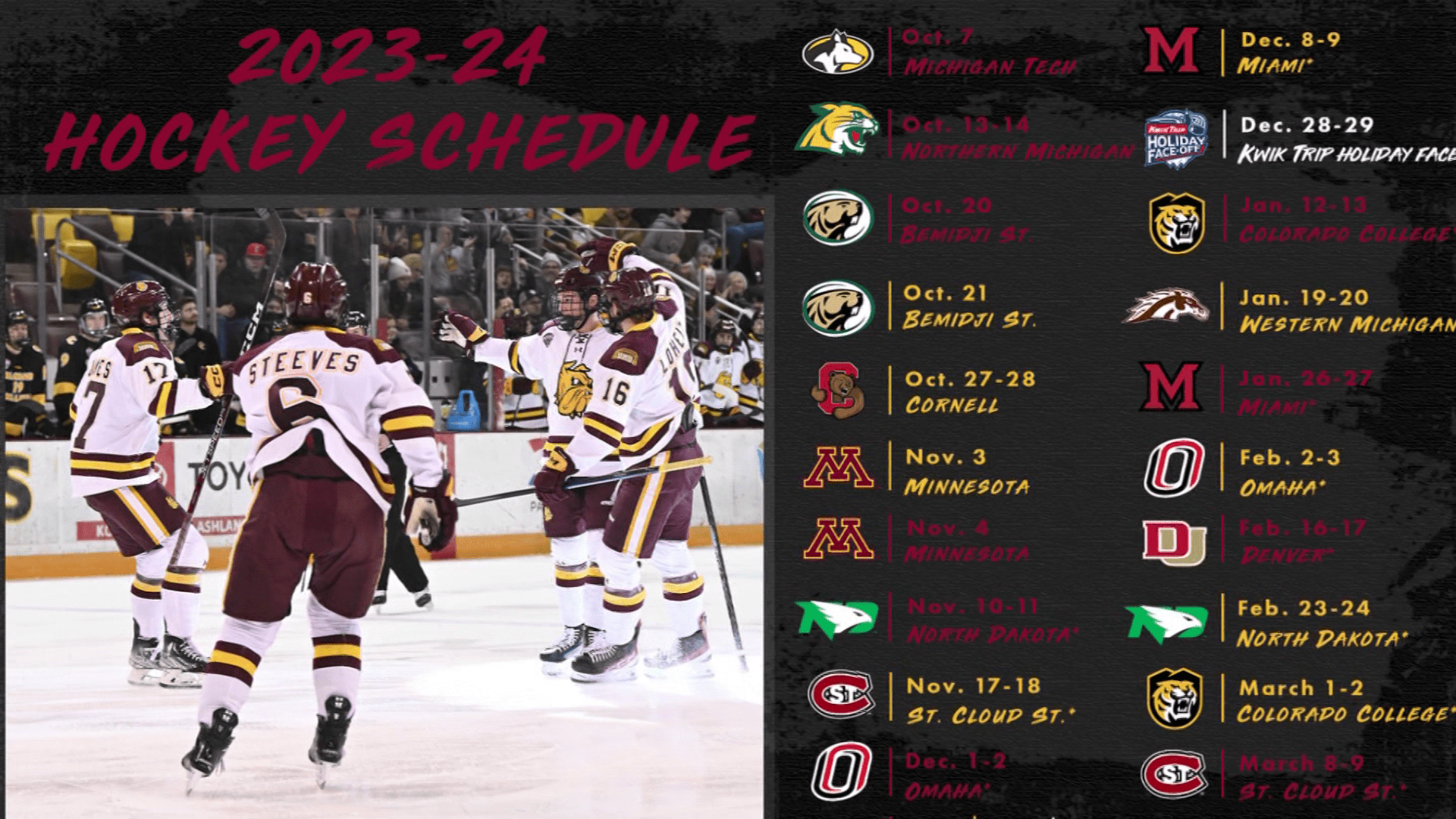
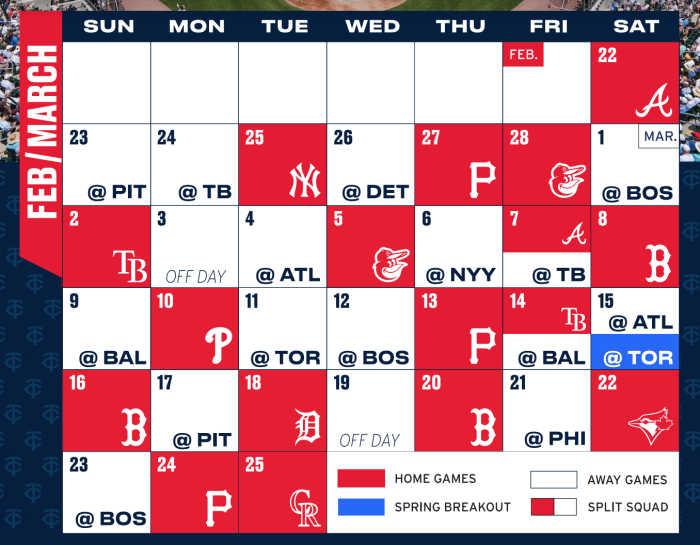
Pharmacies licensed and located in Minnesota must report to the MN PMP all schedule II-V controlled substance prescriptions, along with prescriptions for butalbital and gabapentin, when dispensed. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Table 1 Summary of Gabapentin Policy Changes 2013–2018 State Policy start date Gabapentin policy type Kansas 7/25/2017 PDMP Reporting for Gabapentin Kentucky 3/3/2017 Schedule V Classification of Gabapentin Massachusetts 8/1/2017 PDMP Reporting for Gabapentin Minnesota 8/1/2016 PDMP Reporting for Gabapentin Nebraska 1/1/2018 PDMP Reporting Minnesota Controlled Substances Schedule II-V, butalbital, and gabapentin prescriptions reported 27,586 1,834,360 Pharmacies Reporting 1,635 Generic NameGeneric Name RX Count Q2RX Count Q2 Lorazepam 114,145 lonazepam 86,295 Alprazolam 69,235 iazepam 28,785 Temazepam 11,205 RX Count Q2 Hydrocodone/ Acetaminophen 137,016 Oxycodone HL 130,330 because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). There are established five schedules of controlled substances, to be known as Schedules I, II, III, IV, and V. The schedules consist of the substances listed in this section by whatever official name, common or usual name, chemical name, or trade name designated. Subd. 2. Schedule I. Gabapentin and the Minnesota PMP On August 1, 2016, a law went into effect that requires dispens-ers to report the dispensing of gabapentin to the PMP. Gabapen - tin is not a scheduled controlled substance (CS) in the state of Minnesota. However, per Minnesota Statute §152.126, gabapentin must be reported to the PMP. Gabapentin closely resembles pregabalin, a Schedule V controlled substance, in its chemical structure and pharmaceological activity. Gabapentin is a prescription medication approved by the Federal Food and Drug Administration for the treatment of neuropathic pain and epileptic disorders. For the purposes of this section, controlled substances includes butalbital and gabapentin. (d) "Dispense" or "dispensing" has the meaning given in section 151.01, subdivision 30 . Dispensing does not include the direct administering of a controlled substance to a patient by a licensed health care professional. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. In general, medications classified as Schedule V (Schedule 5) controlled substances are considered to have the lowest potential for abuse compared to other In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death. 24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Gabapentin enacarbil available under the trade name Horizant is the only gabapentin product approved for treatment of Restless Legs Syndrome (RLS). A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose, but caused an increase in adverse reactions. (Schedules II-V, butalbital and gabapentin) dispensed at this pharmacy are reported to the Minnesota Prescription Monitoring Program as required by Minnesota Statutes Section 152.126 and may be used for program administration purposes. www.pmp.pharmacy.state.mn.us syrups containing codeine are Schedule V controlled substances under federal law, but are Schedule III controlled substances under Minnesota law. However, Minnesota laws regulating controlled substances are primarily based on federal law, and in many instances, incorporate language from the CSA. Controlled Substance Schedules Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Minnesota Controlled Substances Schedule II-V, butalbital, and gabapentin Minnesota Prescription Monitoring Program minnesota.pmp@state.mn.us 651.201.2836 A prescription need not bear a federal drug enforcement administration registration number that authorizes the prescriber to prescribe controlled substances if the drug prescribed is not a controlled substance in Schedule II, III, IV, or V. Dispensers (pharmacies) licensed by the MN Board of Pharmacy must report daily, all Minnesota schedule II-V controlled substances, butalbital and gabapentin prescriptions to the Minnesota Prescription Monitoring Program (MN PMP), when dispensed in or into the State.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |