Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
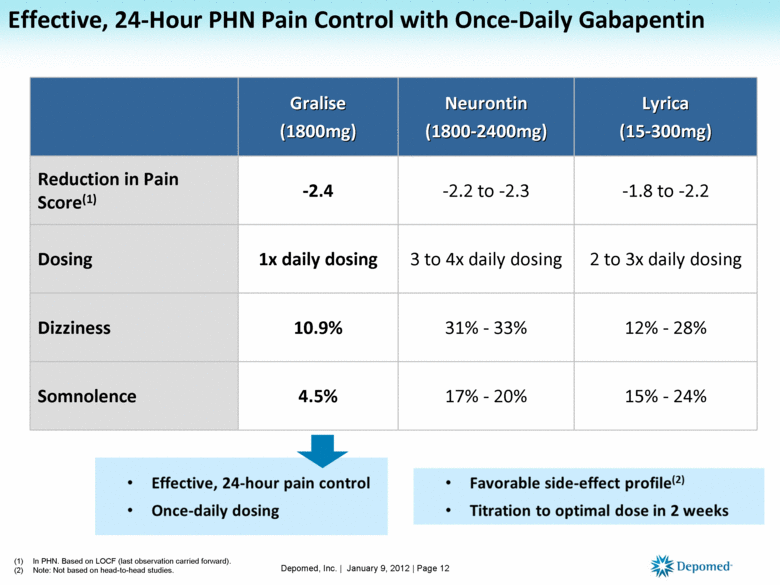
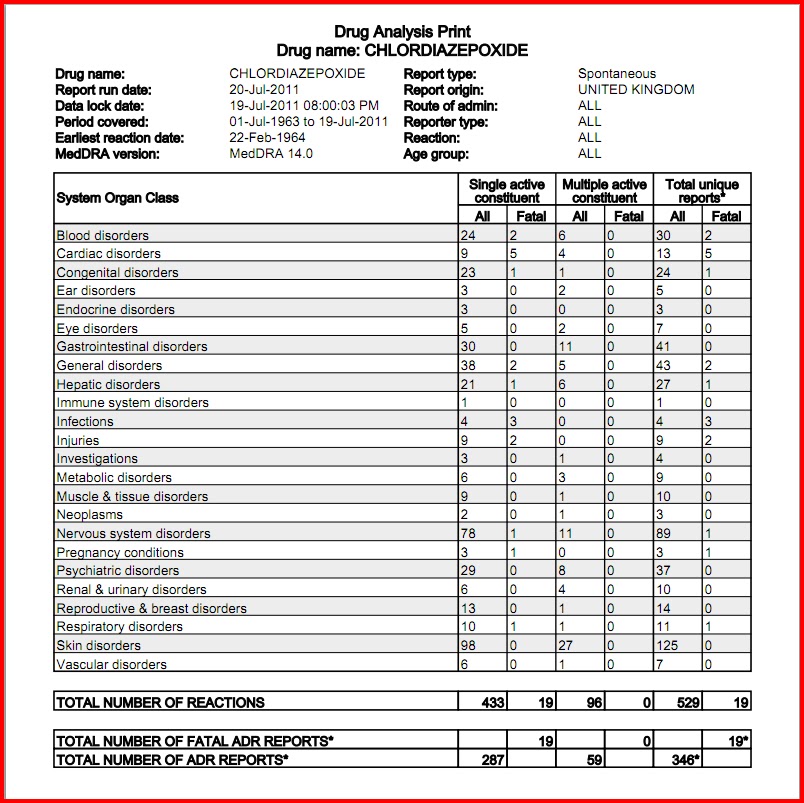
Gabapentin and the Minnesota PMP On August 1, 2016, a law went into effect that requires dispens-ers to report the dispensing of gabapentin to the PMP. Gabapen - tin is not a scheduled controlled substance (CS) in the state of Minnesota. However, per Minnesota Statute §152.126, gabapentin must be reported to the PMP. Pharmacies licensed and located in Minnesota must report to the MN PMP all schedule II-V controlled substance prescriptions, along with prescriptions for butalbital and gabapentin, when dispensed. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Minnesota Controlled Substances Schedule II-V, butalbital, and gabapentin Minnesota Prescription Monitoring Program minnesota.pmp@state.mn.us 651.201.2836 For the purposes of this section, controlled substances includes butalbital and gabapentin. (d) "Dispense" or "dispensing" has the meaning given in section 151.01, subdivision 30. Dispensing does not include the direct administering of a controlled substance to a patient by a licensed health care professional. Subd. 2. Prescription requirements for Schedule III or IV controlled substances. (a) Except as provided in paragraph (b), no person may dispense a controlled substance included in Schedule III or IV of section 152.02 without a prescription issued, as permitted under subdivision 1, by a doctor of medicine, a doctor of osteopathic medicine licensed to practice medicine, a doctor of dental Dispensers (pharmacies) licensed by the Board of Pharmacy must report daily, all MN schedule II-V controlled substances, butalbital and gabapentin prescriptions to the MN PMP when dispensed in or into the State. Minnesota Statutes Section 152.126 governs data collection, retention, and access to the Minnesota Prescription Monitoring Program database. Within the statute there are requirements related to the MN PMP. Please click below to review the full statute language, or each requirement for details. MN Statutes § 152.126 We would like to show you a description here but the site won’t allow us. In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death. 24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in Dispensers (pharmacies) licensed by the MN Board of Pharmacy must report daily, all Minnesota schedule II-V controlled substances, butalbital and gabapentin prescriptions to the Minnesota Prescription Monitoring Program (MN PMP), when dispensed in or into the State. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- 152.02 SCHEDULES OF CONTROLLED SUBSTANCES; ADMINISTRATION OF CHAPTER. Subdivision 1. Five schedules. There are established five schedules of controlled substances, to be known as Schedules I, II, III, IV, and V. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). (Schedules II-V, butalbital and gabapentin) dispensed at this pharmacy are reported to the Minnesota Prescription Monitoring Program as required by Minnesota Statutes Section 152.126 and may be used for program administration purposes. www.pmp.pharmacy.state.mn.us Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: Gabapentin and the Minnesota PMP On August 1, 2016, a law went into effect that requires dispens-ers to report the dispensing of gabapentin to the PMP. Gabapen - tin is not a scheduled controlled substance (CS) in the state of Minnesota. However, per Minnesota Statute §152.126, gabapentin must be reported to the PMP. I have received e-mail (spam) and/or a fax from a company claiming to be a pharmacy licensed by the Minnesota Board of Pharmacy. (Or, I have visited a website that displays a license supposedly issued by the Board).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |