Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
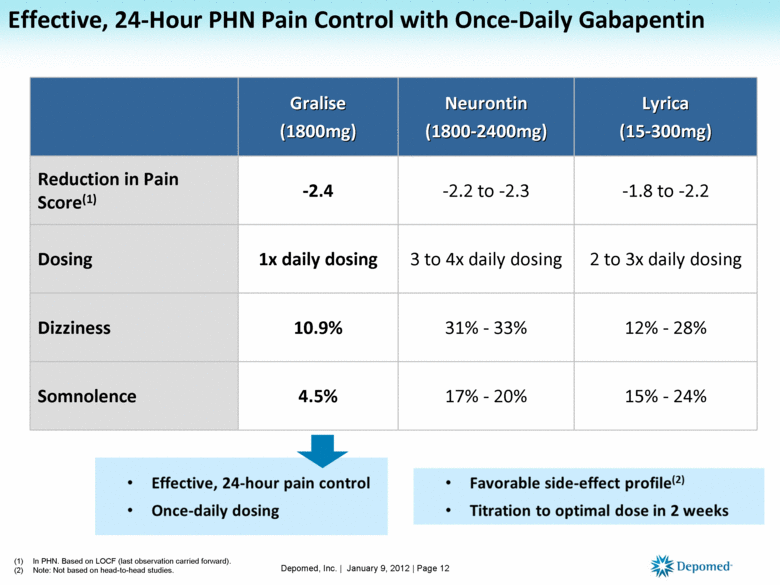
Overseen by the NJ Division of Consumer Affairs Statewide electronic database for collecting data from outpatient pharmacies on: Controlled Dangerous Substances (CDS) Schedules II, III, IV and V Human Growth Hormone Products Gabapentin (May 7, 2018) Operational since September 1, 2011 Rules and regulations of the New Jersey Division of Consumer Affairs (Division), the boards and committees in, and other units of, the Division are codified in Title 13 of the New Jersey Administrative Code, published by LexisNexis. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Pharmacies must report specific information on all transactions for prescriptions of Schedule II, III, IV, and V Dangerous Substances (CDS) as well as Human Growth Hormone (HGH) and gabapentin. The information must be entered via the secure NJPMP website and must include, among other things: the patient's full name and date of birth; the permit A pharmacy filling prescriptions in New Jersey in an outpatient setting for a Schedule II, III, IV, or V controlled dangerous substance, for human growth hormone, or gabapentin. i. For purposes of this subchapter, "human growth hormone" means somatrem, somatropin, or any analogue of either of them, consistent with 21 U.S.C. § 333 (e)4; 2. All intent to distribute charges handled at the Superior Court in New Brunswick involve “scheduled” controlled dangerous substances, also known as CDS. A controlled dangerous substance is a drug that has been classified under New Jersey law in one of five (5) schedules. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death. 24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- On May 7, 2018, the New Jersey Division of Consumer Affairs began requiring pharmacies begin reporting prescriptions for the unscheduled drug gabapentin to the New Jersey Prescription Monitoring Program (PMP). The drug, known under brand names Neurontin and Gralise, is currently unscheduled. 1) A pharmacy filling prescriptions in New Jersey in an outpatient setting for a Schedule II, III, IV, or V controlled dangerous substance, for human growth hormone, or gabapentin. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Pursuant to N.J.S.A. 45:1-45 et. seq., and N.J.A.C. 13:45A-35.3, pharmacies that dispense Schedule II-V Controlled Dangerous Substances (CDS), Human Growth Hormone (HGH), and gabapentin in New Jersey, or into New Jersey, are required to submit data on all transactions for such drugs to the New Jersey Prescription Monitoring Program (NJPMP). TRENTON – Attorney General Gurbir S. Grewal, the Division of Consumer Affairs, and the New Jersey Coordinator for Addiction Responses and Enforcement Strategies ("NJ CARES") today announced enhancements to the NJ Prescription Monitoring Program (“NJ PMP”) that make it easier for prescribers and pharmacists to identify and manage patients at risk for controlled substance abuse and misuse. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. Though, Gabapentin doesn’t bind to receptors as GABA would. The science behind how Gabapentin works is unclear. Research shows that it increases the amount of GABA for the brain to use. Although it doesn’t bind to receptors, the chemicals in Gabapentin produce the same effect overall. It reduces pain and can increase relaxation overall. (a) A pharmacy filling a prescription for a Schedule II, III, IV, or V controlled dangerous substance, [or] for human growth hormone, as defined in N.J.A.C. 13:45A-35.1, or for gabapentin, in an outpatient setting, shall collect and electronically transmit to the Division's PMP vendor on a daily basis information for each prescription, as Nebraska 1/1/2018 PDMP Reporting for Gabapentin New Jersey 5/7/2018 PDMP Reporting for Gabapentin North Dakota 8/1/2017 PDMP Reporting for Gabapentin Ohio 12/1/2016 PDMP Reporting for Gabapentin Tennessee 7/1/2018 Schedule V Classification of Gabapentin Virginia 2/23/2017 PDMP Reporting for Gabapentin West Virginia 6/7/2018 Schedule V Before dispensing a prescribed drug, pharmacists who have registered to use the NJPMP are able to access the NJPMP website and request the CDS and HGH, and gabapentin prescription history of the patient.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |