Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
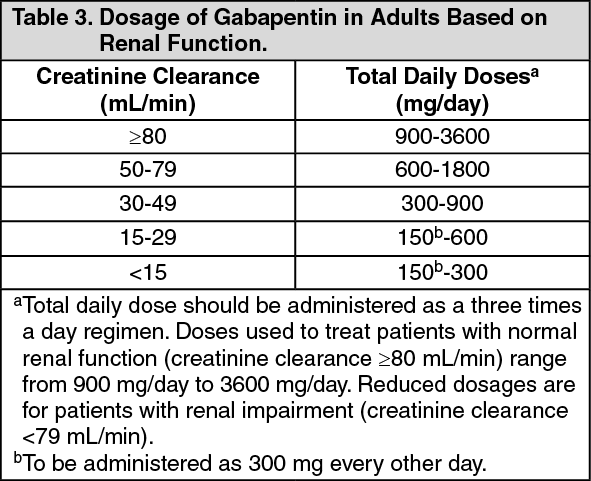
Oklahoma uses the same drug schedules as the Federal Government—I through V. Oklahoma will use the scheduling system for both possession of drugs and possession with intent to distribute through June 30, 2017. On July 1, 2017, these schedules will only apply to possession with intent to distribute. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Schedule 1 No N/A Schedule 2 Yes Within 24 hours or the next business day Schedule 3 Yes Within 24 hours or the next business day Schedule 4 Yes Within 24 hours or the next business day Schedule 5 Yes Within 24 hours or the next business day Schedule 6 Gabapentin Within 24 hours or the next business day To decrease your risk of a drug conviction, avoid the following new additions to Oklahoma’s controlled substance list. The Schedule I and II Drugs Added to the List. The following drugs were added to Oklahoma’s controlled substance list: Schedule I drugs. Between August 2016 and July 2018, three states classified gabapentin as a Schedule V drug and nine states implemented prescription drug monitoring program (PDMP) regulation for gabapentin. It is highly unusual for states to take drug regulation Michael Abrams, MPH, PhD, senior health researcher at Public Citizen’s Health Research Group, discusses whether classification of gabapentin as schedule V would end patients’ access to the drug. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Oklahoma Board of Nursing . 2501 N. Lincoln Blvd., Ste. 207 . Oklahoma City, Oklahoma 73105 . 405-962-1800 . Exclusionary Formulary for Advanced Practice Registered Nurses with Prescriptive Authority . The Advanced Practice Registered Nurse (Certified Nurse Practitioner, Clinical Nurse Specialist and Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin Prescribers are required to check the PMP prior to prescribing opioids, benzodiazepines, and carisoprodol and every 180 days. To access the Oklahoma PMP, please click the icon below. States with a gabapentin schedule change or PDMP regulation enacted before 2019 were included in the intervention group. For the Schedule V DID analysis, a control group of the ten highest opioid-prescribing states was used. complied with the registration requirements of the Oklahoma Uniform Controlled Dangerous Substances Act, in good faith and in the course of professional practice only, may prescribe and administer Schedule III, IV, and V controlled dangerous substances. Oklahoma Board of Nursing 2501 N. Lincoln Blvd., Ste. 207 Oklahoma City, OK 73105 (405) 962-1800 Oklahoma Gov. Kevin Stitt has signed into law bipartisan legislation that amends a state law regulating opioids and other controlled substances to better protect pain patients from forced opioid tapering and dose limits. SB57 was passed unanimously by the Oklahoma House and Senate last month, and signed by Gov. Stitt on Monday. Patient OBN is authorized by Title 63 of the Oklahoma Statutes to enforce the Uniform Controlled Dangerous Substances Act. OBN also has the authority to investigate criminal offenses related to human trafficking and money laundering. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Gabapentin enacarbil available under the trade name Horizant is the only gabapentin product approved for treatment of Restless Legs Syndrome (RLS). A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose, but caused an increase in adverse reactions. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |